Chiroptical Generation, Switching, and Long-Term Memory in Supramolecular Azobenzene-Pendant Polymer: Regulation by Cellulose Peralkyl Esters, D-/L-Glucose Permethyl Esters, Solvents, UV Light Irradiation, and Thermal Annealing Process†
Shiyuan Yi
State and Local Joint Engineering Laboratory for Novel Functional Polymeric Materials, Jiangsu Engineering Laboratory of Novel Functional Polymeric Materials, Suzhou Key Laboratory of Macromolecular Design and Precision Synthesis, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou, Jiangsu, 215123 China
Search for more papers by this authorLaibing Wang
State and Local Joint Engineering Laboratory for Novel Functional Polymeric Materials, Jiangsu Engineering Laboratory of Novel Functional Polymeric Materials, Suzhou Key Laboratory of Macromolecular Design and Precision Synthesis, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou, Jiangsu, 215123 China
Search for more papers by this authorCorresponding Author
Xiaoxiao Cheng
State and Local Joint Engineering Laboratory for Novel Functional Polymeric Materials, Jiangsu Engineering Laboratory of Novel Functional Polymeric Materials, Suzhou Key Laboratory of Macromolecular Design and Precision Synthesis, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou, Jiangsu, 215123 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Michiya Fujiki
Graduate School of Science and Technology, Nara Institute of Science and Technology, 8916-5 Takayama, Ikoma, Nara, 630-0192 Japan
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Wei Zhang
State and Local Joint Engineering Laboratory for Novel Functional Polymeric Materials, Jiangsu Engineering Laboratory of Novel Functional Polymeric Materials, Suzhou Key Laboratory of Macromolecular Design and Precision Synthesis, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou, Jiangsu, 215123 China
School of Chemical and Environmental Engineering, Anhui Polytechnic University, Wuhu, Anhui, 241000 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorShiyuan Yi
State and Local Joint Engineering Laboratory for Novel Functional Polymeric Materials, Jiangsu Engineering Laboratory of Novel Functional Polymeric Materials, Suzhou Key Laboratory of Macromolecular Design and Precision Synthesis, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou, Jiangsu, 215123 China
Search for more papers by this authorLaibing Wang
State and Local Joint Engineering Laboratory for Novel Functional Polymeric Materials, Jiangsu Engineering Laboratory of Novel Functional Polymeric Materials, Suzhou Key Laboratory of Macromolecular Design and Precision Synthesis, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou, Jiangsu, 215123 China
Search for more papers by this authorCorresponding Author
Xiaoxiao Cheng
State and Local Joint Engineering Laboratory for Novel Functional Polymeric Materials, Jiangsu Engineering Laboratory of Novel Functional Polymeric Materials, Suzhou Key Laboratory of Macromolecular Design and Precision Synthesis, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou, Jiangsu, 215123 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Michiya Fujiki
Graduate School of Science and Technology, Nara Institute of Science and Technology, 8916-5 Takayama, Ikoma, Nara, 630-0192 Japan
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Wei Zhang
State and Local Joint Engineering Laboratory for Novel Functional Polymeric Materials, Jiangsu Engineering Laboratory of Novel Functional Polymeric Materials, Suzhou Key Laboratory of Macromolecular Design and Precision Synthesis, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou, Jiangsu, 215123 China
School of Chemical and Environmental Engineering, Anhui Polytechnic University, Wuhu, Anhui, 241000 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorDedicated to the Special Issue of Emerging Themes in Polymer Science.
Comprehensive Summary
Various optically active polymers are known to afford sophisticated chirality-related functionalities, i.e., asymmetric catalysis, chiroptical switching and memory in UV-vis-NIR region, chromatographic separation of enantiomers, and sensors for molecular chirality. Recently, material researchers have paid much attention to the design of chiral supramolecular architectures from achiral polymers upon intermolecular interactions with help of greener biosources. The present article reports an instantaneous generation of ambidextrous supramolecules revealing light-driven chiroptical switching/memory in UV-vis region when achiral azobenzene-containing vinylpolymers are non-covalently interacted with alkyl ester derivatives of natural cellulose and D-/L-glucose. It was recognized that the semi-synthetic biomaterials efficiently work as chirality-inducing scaffoldings to several achiral and optically inactive molecules, oligomers, and polymers. Our successful results shed light on a new approach of how inexpensive poly-/mono-saccharide derivatives can afford supramolecular chiroptical systems with the azobenzene pendant polymer as aggregates in suspension and liquid-crystalline films with minimal energy, time, and cost.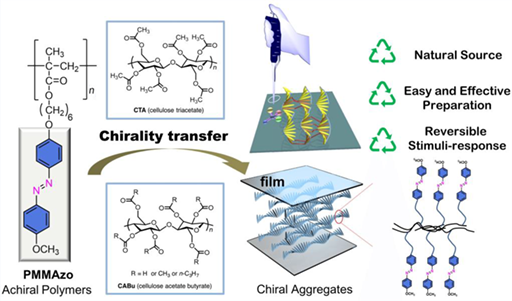
Supporting Information
| Filename | Description |
|---|---|
| cjoc202300333-sup-0001-supinfo.pdfPDF document, 680.3 KB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Zhang, L.; Wang, H. X.; Li, S.; Liu, M. H. Supramolecular chiroptical switches. Chem. Soc. Rev. 2020, 49, 9095–9120.
- 2 Hembury, G. A.; Borovkov, V. V.; Inoue, Y. Chirality-sensing supramolecular systems. Chem. Rev. 2008, 108, 1–73.
- 3 Cornelissen, J. J. L. M.; Rowan, A. E.; Nolte, R. J. M.; Sommerdijk, N. A. J. M. Chiral architectures from macromolecular building blocks. Chem. Rev. 2001, 101, 4039–4070.
- 4 Ikkala, O.; ten Brinke, G. Hierarchical self-assembly in polymeric complexes: Towards functional materials. Chem. Commun. 2004, 2131–2137.
- 5 Liu, M. H.; Zhang, L.; Wang, T. Y. Supramolecular Chirality in Self-Assembled Systems. Chem. Rev. 2015, 115, 7304–7397.
- 6 Lohr, A.; Lysetska, M.; Wurthner, F. Supramolecular stereomutation in kinetic and thermodynamic self-assembly of helical merocyanine dye nanorods. Angew. Chem. Int. Ed. 2005, 44, 5071–5074.
- 7 Cheng, X. X.; Miao, T. F.; Yin, L.; Ji, Y. J.; Li, Y. Y.; Zhang, Z. B.; Zhang, W.; Zhu, X. L. In Situ Controlled Construction of a Hierarchical Supramolecular Chiral Liquid-Crystalline Polymer Assembly. Angew. Chem. Int. Ed. 2020, 59, 9669–9677.
- 8 Zhang, L.; Qin, L.; Wang, X. F.; Cao, H.; Liu, M. H. Supramolecular Chirality in Self-Assembled Soft Materials: Regulation of Chiral Nanostructures and Chiral Functions. Adv. Mater. 2014, 26, 6959–6964.
- 9 Zou, X. W.; Ye, H. C.; Zhang, S. W.; Liu, Y.; Wang, C. Y.; Cheng, Y. X. Synthesis and asymmetric enantioselective addition of chiral polybinaphthyl and polybinaphthol ligands. Chin. J. Chem. 2007, 25, 992–997.
- 10 Guo, Q.; Wang, J. C.; Zhu, L. Y.; Wei, Z. X. Modulating the Helicity of Sugar-Substituted Perylene Diimide Self-assemblies by Solvent Polarilities. Chin. J. Chem. 2015, 33, 95–100.
- 11
Huang, S.; Yu, H. F.; Li, Q. Supramolecular Chirality Transfer toward Chiral Aggregation: Asymmetric Hierarchical Self-Assembly. Adv. Sci. 2021, 8, 2002132.
10.1002/advs.202002132 Google Scholar
- 12 Xing, P. Y.; Zhao, Y. L. Controlling Supramolecular Chirality in Multicomponent Self-Assembled Systems. Acc. Chem. Res. 2018, 51, 2324–2334.
- 13 Palmans, A. R. A.; Meijer, E. W. Amplification of chirality in dynamic supramolecular aggregates. Angew. Chem. Int. Ed. 2007, 46, 8948–8968.
- 14 He, Z. X.; Miao, T. F.; Cheng, X. X.; Ma, H. T.; Ma, Y. F.; Zhang, W.; Zhu, X. L. Building permanently optically active particles from an absolutely achiral polymer. Polym. Chem. 2022, 13, 1953–1959.
- 15 Gan, Y. J.; Dai, H. B.; Ma, Y. F.; Cheng, X. X.; Wang, Z.; Zhang, W. Regulating Chiral Helical Structures in Liquid-Crystalline Block Copolymers with Chiroptical Response by Synergistic Asymmetric Effects. Macromolecules 2022, 55, 8556–8565.
- 16 Miao, T. F.; Cheng, X. X.; Ma, H. T.; Zhang, W.; Zhu, X. L. Induction, fixation and recovery of self-organized helical superstructures in achiral liquid crystalline polymer. Polym. Chem. 2021, 12, 5931–5936.
- 17 Jiang, F.; Qu, J. Q.; Chen, H. Q. Synthesis and Characterization of Poly(chiral methylpropargyl ester)s Carrying Azobenzene Moieties. Chin. J. Chem. 2009, 27, 2079–2084.
- 18 Wang, S.; Feng, X. Y.; Zhang, J.; Wan, X. H. Doublet Chirality Transfer and Reversible Helical Transition in Poly(3,5-disubstituted phenylacetylene)s with Pyrene as a Probe Unit(dagger). Chin. J. Chem. 2020, 38, 570–576.
- 19 Ohira, A.; Okoshi, K.; Fujiki, M.; Kunitake, M.; Naito, M.; Hagihara, T. Versatile helical polymer films: Chiroptical inversion switching and memory with re-writable (RW) and write-once read-many (WORM) modes. Adv. Mater. 2004, 16, 1645–1650.
- 20 Bisoyi, H. K.; Li, Q. Light-Directed Dynamic Chirality Inversion in Functional Self-Organized Helical Superstructures. Angew. Chem. Int. Ed. 2016, 55, 2994–3010.
- 21 Miao, T. F.; Cheng, X. X.; Qian, Y. L.; Zhuang, Y. L.; Zhang, W. Engineering Achiral Liquid Crystalline Polymers for Chiral Self-Recovery. Int. J. Mol. Sci. 2021, 22, 11980.
- 22 Ikeda, M.; Hasegawa, T.; Numata, M.; Sugikawa, K.; Sakurai, K.; Fujiki, M.; Shinkai, S. Instantaneous inclusion of a polynucleotide and hydrophobic guest molecules into a helical core of cationic beta-1,3- glucan polysaccharide. J. Am. Chem. Soc. 2007, 129, 3979–3988.
- 23 Guo, S. B.; Suzuki, N.; Fujiki, M. Oligo- and Polyfluorenes Meet Cellulose Alkyl Esters: Retention, Inversion, and Racemization of Circularly Polarized Luminescence (CPL) and Circular Dichroism (CD) via Intermolecular C-H/O=C Interactions. Macromolecules 2017, 50, 1778–1789.
- 24 Abe, M.; Fukaya, Y.; Ohno, H. Fast and facile dissolution of cellulose with tetrabutylphosphonium hydroxide containing 40 wt% water. Chem. Commun. 2012, 48, 1808–1810.
- 25 Guo, S. B.; Kamite, H.; Suzuki, N.; Wang, L. B.; Ohkubo, A.; Fujiki, M., Ambidextrous Chirality Transfer Capability from Cellulose Tris(phenylcarbamate) to Nonhelical Chainlike Luminophores: Achiral Solvent-Driven Helix-Helix Transition of Oligo- and Polyfluorenes Revealed by Sign Inversion of Circularly Polarized Luminescence and Circular Dichroism Spectra. Biomacromolecules 2018, 19, 449–459.
- 26 Stipanovic, A. J.; Sarko, A. Molecular and Crystal-Structure of Cellulose Triacetate-L-Parallel Chain Structure. Polymer 1978, 19, 3–8.
- 27 Fujiki, M.; Wang, L. B.; Ogata, N.; Asanoma, F.; Okubo, A.; Okazaki, S.; Kamite, H.; Jalilah, A. Chirogenesis and Pfeiffer Effect in Optically Inactive Eu(III)and Tb-III Tris(beta-diketonate) Upon Intermolecular Chirality Transfer From Poly- and Monosaccharide Alkyl Esters and alpha-Pinene: Emerging Circularly Polarized Luminescence (CPL) and Circular Dichroism (CD). Front. Chem. 2020, 8, 685.
- 28 Jiang, S. Q.; Zhao, Y.; Wang, L. B.; Yin, L.; Zhang, Z. B.; Zhu, J.; Zhang, W.; Zhu, X. L. Photocontrollable Induction of Supramolecular Chirality in Achiral Side Chain Azo-Containing Polymers through Preferential Chiral Solvation. Polym. Chem. 2015, 6, 4230–4239.
- 29 Cheng, X. X.; Gan, Y. J.; Zhang, G.; Song, Q. P.; Zhang, Z. B.; Zhang, W. Conformationally supramolecular chirality prevails over configurational point chirality in side-chain liquid crystalline polymers. Chem. Sci. 2023, 14, 5116–5124.
- 30 Zhao, Y.; Chen, H. L.; Yin, L.; Cheng, X. X.; Zhang, W.; Zhu, X. L. Chirality induction of achiral polydialkylfluorenes by chiral solvation: odd-even and side chain length dependence. Polym. Chem. 2018, 9, 2295–2301.
- 31 Qing, G. Y.; Shan, X. X.; Chen, W. R.; Lv, Z. Y.; Xiong, P.; Sun, T. L. Solvent-Driven Chiral-Interaction Reversion for Organogel Formation. Angew. Chem. Int. Ed. 2014, 53, 2124–2129.
- 32 Zhang, W.; Yoshida, K.; Fujiki, M.; Zhu, X. L. Unpolarized-Light-Driven Amplified Chiroptical Modulation Between Chiral Aggregation and Achiral Disaggregation of an Azobenzene-alt-Fluorene Copolymer in Limonene. Macromolecules 2011, 44, 5105–5111.
- 33 Yashima, E.; Maeda, K. Chirality-Responsive Helical Polymers. Macromolecules 2008, 41, 3–12.
- 34 Zhang, Y. J.; Deng, J. P.; Pan, K. Chiral Helical Polymer Nanomaterials with Tunable Morphology: Prepared with Chiral Solvent to Induce Helix-Sense-Selective Precipitation Polymerization. Macromolecules 2018, 51, 8878–8886.
- 35 Miao, T. F.; Cheng, X. X.; Ma, H. T.; He, Z. X.; Zhang, Z. B.; Zhou, N. A. C.; Zhang, W.; Zhu, X. L. Transfer, Amplification, Storage, and Complete Self-Recovery of Supramolecular Chirality in an Achiral Polymer System. Angew. Chem. Int. Ed. 2021, 60, 18566–18571.




