Bio-inspired Small Molecular Catalysis
Mao Cai
Sinopec Beijing Research Institute of Chemical Industry, Beijing, 100013 China
Search for more papers by this authorRunze Zhang
Center of Basic Molecular Science, Department of Chemistry, Tsinghua University, Beijing, 100084 China
Search for more papers by this authorChunming Yang
Center of Basic Molecular Science, Department of Chemistry, Tsinghua University, Beijing, 100084 China
Search for more papers by this authorCorresponding Author
Sanzhong Luo
Center of Basic Molecular Science, Department of Chemistry, Tsinghua University, Beijing, 100084 China
E-mail: [email protected]Search for more papers by this authorMao Cai
Sinopec Beijing Research Institute of Chemical Industry, Beijing, 100013 China
Search for more papers by this authorRunze Zhang
Center of Basic Molecular Science, Department of Chemistry, Tsinghua University, Beijing, 100084 China
Search for more papers by this authorChunming Yang
Center of Basic Molecular Science, Department of Chemistry, Tsinghua University, Beijing, 100084 China
Search for more papers by this authorCorresponding Author
Sanzhong Luo
Center of Basic Molecular Science, Department of Chemistry, Tsinghua University, Beijing, 100084 China
E-mail: [email protected]Search for more papers by this authorComprehensive Summary
Nature is an inspiring resource for chemists to exploit uncharted chemical spaces. Enzymes in nature are characteristic of high efficiency and exquisite stereocontrol under rather mild conditions. Not surprisingly, chemists have a long history in developing new catalysts by learning from and imitating the natural enzymes. This review summarizes our long-standing efforts in developing bio-inspired small molecular catalysis. These were categorized according to the employed strategies: 1) bi-/multi-functional strategy for the bio-inspired chiral primary amine catalyst; 2) cofactor strategy for bio-inspired ortho-quinone catalyst; 3) intermediate strategy for chiral latent carbocation catalysis based on natural carbocation process. Each section includes brief introduction of the biological origin, design of catalyst, development of methodology as well as the underlying mechanistic insights.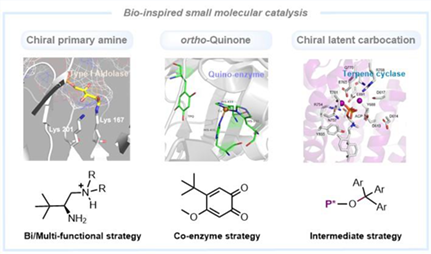
What is the most favorite and original chemistry developed in your research group?
I hope it's always the next one.
How do you get into this specific field? Could you please share some experiences with our readers?
Nature provides many astonishing catalytic machineries for building up molecular complexity and harnessing energy in the most efficient ways. Nature's recipe for catalysis serves as a starting point to develop new catalyst for synthetic and energy chemistry. I was trained as a synthetic chemist in a physical organic group in Nankai and ICCAS, and spent part of my graduate studies doing enzymatic synthesis of oligosaccharide and its related biological applications in Ohio State University. This gave me a mixed view on organocatalysis, then under its golden stage of renaissance, not only for its synthetic potentials as a new catalytic concept, but also with an eye on its biological origin and possible impact beyond
synthesis. I also benefit a lot from the physical organic chemistry training which helps to shape my research path in width and depth, in a way I never imagined and expected at the beginning of my career.
How do you supervise your students?
Try to inspire beyond instruct. I learned and am still learning. “因材施教” is easy to say than to do. In reality, I become bossy the way I dislike and have to instruct heavily.
What is the most important personality for scientific research?
Curiosity, broad reading and sufficient “free time” for daydreaming. In order to have enough time to waste, you have to work really hard at first.
Who influences you mostly in your life?
My Ph.D. supervisor, Prof. Jin-Pei Cheng who teaches me a rational and quantitative view for chemistry and inspires me all the time to pursue a rational approach in research.
References
- 1 Fischer, E. Syntheses in the Purine and Sugar Group. In Nobel Lectures, Chemistry 1901—1921, Elsevier, Amsterdam, 1966, pp. 21–35.
- 2 Dub, P. A.; Gordon, J. C. The Role of the Metal-Bound N–H Functionality in Noyori-Type Molecular Catalysts. Nat. Rev. Chem. 2018, 2, 396–408.
- 3 Xie, J.-H.; Liu, X.-Y.; Xie, J.-B.; Wang, L.-X.; Zhou, Q.-L. An Additional Coordination Group Leads to Extremely Efficient Chiral Iridium Catalysts for Asymmetric Hydrogenation of Ketones. Angew. Chem. Int. Ed. 2011, 50, 7329–7332.
- 4 Yang, Y.; Arnold, F. H. Navigating the Unnatural Reaction Space: Directed Evolution of Heme Proteins for Selective Carbene and Nitrene Transfer. Acc. Chem. Res. 2021, 54, 1209–1225.
- 5 Mukherjee, S.; Yang, J. W.; Hoffmann, S.; List, B. Asymmetric Enamine Catalysis. Chem. Rev. 2007, 107, 5471–5569.
- 6 Flanigan, D. M.; Romanov-Michailidis, F.; White, N. A.; Rovis, T. Organocatalytic Reactions Enabled by N-Heterocyclic Carbenes. Chem. Rev. 2015, 115, 9307–9387.
- 7 Chen, X.-Y.; Gao, Z.-H.; Ye, S. Bifunctional N-Heterocyclic Carbenes Derived from l-Pyroglutamic Acid and Their Applications in Enantioselective Organocatalysis. Acc. Chem. Res. 2020, 53, 690–702.
- 8 Xie, Y.; Pan, H.; Liu, M.; Xiao, X.; Shi, Y. Progress in Asymmetric Biomimetic Transamination of Carbonyl Compounds. Chem. Soc. Rev. 2015, 44, 1740–1748.
- 9 Chen, J.; Liu, Y. E.; Gong, X.; Shi, L.; Zhao, B. Biomimetic Chiral Pyridoxal and Pyridoxamine Catalysts. Chin. J. Chem. 2018, 37, 103–112.
- 10Dalko, P. I. Enantioselective Organocatalysis: Reactions and Experimental Procedures, Wiley-VCH, Weinheim, Germany, 2007.
- 11 List, B.; Lerner, R. A.; Barbas, C. F. Proline-Catalyzed Direct Asymmetric Aldol Reactions. J. Am. Chem. Soc. 2000, 122, 2395–2396.
- 12 Ahrendt, K. A.; Borths, C. J.; MacMillan, D. W. C. New Strategies for Organic Catalysis: The First Highly Enantioselective Organocatalytic Diels−Alder Reaction. J. Am. Chem. Soc. 2000, 122, 4243–4244.
- 13 Fessner, W.-D. Aldolases: Enzymes for Making and Breaking C-C Bonds. In Asymmetric Organic Synthesis with Enzymes, Wiley-VCH, Germany, Weinheim, 2008, pp. 275–318.
- 14 Hajos, Z. G.; Parrish, D. R. Asymmetric Synthesis of Bicyclic Intermediates of Natural Product Chemistry. J. Org. Chem. 1974, 39, 1615–1621.
- 15 Hine, J. Bifunctional Catalysis of α-Hydrogen Exchange of Aldehydes and Ketones. Acc. Chem. Res. 1978, 11, 1–7.
- 16 Ishihara, K.; Nakano, K. Design of an Organocatalyst for the Enantioselective Diels−Alder Reaction with α-Acyloxyacroleins. J. Am. Chem. Soc. 2005, 127, 10504–10505.
- 17 Ibrahem, I.; Zou, W.; Engqvist, M.; Xu, Y.; Córdova, A. Acyclic Chiral Amines and Amino Acids as Inexpensive and Readily Tunable Catalysts for the Direct Asymmetric Three-Component Mannich Reaction. Chem. - Eur. J. 2005, 11, 7024–7029.
- 18 Córdova, A.; Zou, W.; Ibrahem, I.; Reyes, E.; Engqvist, M.; Liao, W.-W. Acyclic Amino Acid-Catalyzed Direct Asymmetric Aldol Reactions: Alanine, the Simplest Stereoselective Organocatalyst. Chem. Commun. 2005, 3586–3588.
- 19 Tsogoeva, S. B.; Wei, S. Highly Enantioselective Addition of Ketones to Nitroolefins Catalyzed by New Thiourea–Amine Bifunctional Organocatalysts. Chem. Commun. 2006, 1451–1453.
- 20 Huang, H.; Jacobsen, E. N. Highly Enantioselective Direct Conjugate Addition of Ketones to Nitroalkenes Promoted by a Chiral Primary Amine−Thiourea Catalyst. J. Am. Chem. Soc. 2006, 128, 7170–7171.
- 21 Jiang, Z.; Liang, Z.; Wu, X.; Lu, Y. Asymmetric Aldol Reactions Catalyzed by Tryptophan in Water. Chem. Commun. 2006, 2801–2803.
- 22 Xu, L.-W.; Lu, Y. Primary Amino Acids: Privileged Catalysts in Enantioselective Organocatalysis. Org. Biomol. Chem. 2008, 6, 2047–2053.
- 23 Xu, L.-W.; Luo, J.; Lu, Y. Asymmetric Catalysis with Chiral Primary Amine-Based Organocatalysts. Chem. Commun. 2009, 1807–1821.
- 24 Wang, N.; Wu, Z.; Wang, J.; Ullah, N.; Lu, Y. Recent Applications of Asymmetric Organocatalytic Annulation Reactions in Natural Product Synthesis. Chem. Soc. Rev. 2021, 50, 9766–9793.
- 25 Chen, Z.-C.; Du, W.; Chen, Y.-C. New Amines and Activation Modes in Asymmetric Aminocatalysis. Chin. J. Chem. 2021, 39, 1775–1786.
- 26 Luo, S.; Xu, H.; Li, J.; Zhang, L.; Cheng, J.-P. A Simple Primary−Tertiary Diamine−Brønsted Acid Catalyst for Asymmetric Direct Aldol Reactions of Linear Aliphatic Ketones. J. Am. Chem. Soc. 2007, 129, 3074–3075.
- 27 Luo, S.; Zhang, L. Bio-Inspired Chiral Primary Amine Catalysis. Synlett 2012, 23, 1575–1589.
- 28 Zhang, L.; Fu, N.; Luo, S. Pushing the Limits of Aminocatalysis: Enantioselective Transformations of α-Branched β-Ketocarbonyls and Vinyl Ketones by Chiral Primary Amines. Acc. Chem. Res. 2015, 48, 986–997.
- 29 Hu, S.; Li, J.; Xiang, J.; Pan, J.; Luo, S.; Cheng, J.-P. Asymmetric Supramolecular Primary Amine Catalysis in Aqueous Buffer: Connections of Selective Recognition and Asymmetric Catalysis. J. Am. Chem. Soc. 2010, 132, 7216–7228.
- 30 Luo, S.; Zheng, X.; Cheng, J.-P. Asymmetric Bifunctional Primary Aminocatalysis on Magnetic Nanoparticles. Chem. Commun. 2008, 5719–5721.
- 31 Zhang, L.; Luo, S.; Cheng, J.-P. Non-Covalent Immobilization of Asymmetric Organocatalysts. Catal. Sci. Technol. 2011, 1, 507–516.
- 32 Zhang, Q.; Cui, X.; Zhang, L.; Luo, S.; Wang, H.; Wu, Y. Redox Tuning of a Direct Asymmetric Aldol Reaction. Angew. Chem. Int. Ed. 2015, 54, 5210–5213.
- 33 Wang, Y.; Chai, J.; You, C.; Zhang, J.; Mi, X.; Zhang, L.; Luo, S. π-Coordinating Chiral Primary Amine/Palladium Synergistic Catalysis for Asymmetric Allylic Alkylation. J. Am. Chem. Soc. 2020, 142, 3184–3195.
- 34 Zhu, Y.; Zhang, L.; Luo, S. Asymmetric α-Photoalkylation of β-Ketocarbonyls by Primary Amine Catalysis: Facile Access to Acyclic All-Carbon Quaternary Stereocenters. J. Am. Chem. Soc. 2014, 136, 14642–14645.
- 35 Zhang, W.; Zhu, Y.; Zhang, L.; Luo, S. Asymmetric α-Alkylation of β-Ketocarbonyls via Direct Phenacyl Bromide Photolysis by Chiral Primary Amine. Chin. J. Chem. 2018, 36, 716–722.
- 36 Fu, N.; Li, L.; Yang, Q.; Luo, S. Catalytic Asymmetric Electrochemical Oxidative Coupling of Tertiary Amines with Simple Ketones. Org. Lett. 2017, 19, 2122–2125.
- 37 Zhou, H.; Zhang, L.; Xu, C.; Luo, S. Chiral Primary Amine/Palladium Dual Catalysis for Asymmetric Allylic Alkylation of β-Ketocarbonyl Compounds with Allylic Alcohols. Angew. Chem. Int. Ed. 2015, 54, 12645–12648.
- 38 Zhou, H.; Wang, Y.; Zhang, L.; Cai, M.; Luo, S. Enantioselective Terminal Addition to Allenes by Dual Chiral Primary Amine/Palladium Catalysis. J. Am. Chem. Soc. 2017, 139, 3631–3634.
- 39 Xu, C.; Zhang, L.; Luo, S. Merging Aerobic Oxidation and Enamine Catalysis in the Asymmetric α-Amination of β-Ketocarbonyls Using N-hydroxycarbamates as Nitrogen Sources. Angew. Chem. Int. Ed. 2014, 53, 4149–4153.
- 40 Cai, M.; Xu, K.; Li, Y.; Nie, Z.; Zhang, L.; Luo, S. Chiral Primary Amine/Ketone Cooperative Catalysis for Asymmetric α-Hydroxylation with Hydrogen Peroxide. J. Am. Chem. Soc. 2021, 143, 1078–1087.
- 41 Zhang, Q.; Li, Y.; Zhang, L.; Luo, S. Catalytic Asymmetric Disulfuration by a Chiral Bulky Three-Component Lewis Acid-Base. Angew. Chem. Int. Ed. 2021, 60, 10971–10976.
- 42 You, Y.; Zhang, L.; Cui, L.; Mi, X.; Luo, S. Catalytic Asymmetric Mannich Reaction with N-Carbamoyl Imine Surrogates of Formaldehyde and Glyoxylate. Angew. Chem. Int. Ed. 2017, 56, 13814–13818.
- 43 You, Y.; Luo, S. Catalytic Asymmetric Mannich Type Reaction with Tri-/Difluoro- or Trichloroacetaldimine Precursors. Org. Lett. 2018, 20, 7137–7140.
- 44 Leonard, P. M.; Grogan, G. Structure of 6-Oxo Camphor Hydrolase H122A Mutant Bound to Its Natural Product, (2S,4S)-α-Campholinic Acid: Mutant Structure Suggests an Atipical Mode of Transition State Binding for a Crotonase Homolog. J. Biol. Chem. 2004, 279, 31312–31317.
- 45
Grogan, G.; Graf, J.; Jones, A.; Parsons, S.; Turner, N. J.; Flitsch, S. L. An Asymmetric Enzyme-Catalyzed Retro-Claisen Reaction for the Desymmetrization of Cyclic β-Diketones. Angew. Chem. Int. Ed. 2001, 40, 1111–1114.
10.1002/1521-3773(20010316)40:6<1111::AID-ANIE11110>3.0.CO;2-2 CAS PubMed Web of Science® Google Scholar
- 46 Zhu, Y.; Zhang, L.; Luo, S. Asymmetric Retro-Claisen Reaction by Chiral Primary Amine Catalysis. J. Am. Chem. Soc. 2016, 138, 3978–3981.
- 47 Han, Y.; Zhang, L.; Luo, S. Asymmetric Retro-Claisen Reaction by Synergistic Chiral Primary Amine/Palladium Catalysis. Org. Lett. 2019, 21, 7258–7261.
- 48 Han, Y.; Zhang, L.; Luo, S. Highly Stereoselective Construction of β,β-Diaryl-α-Branched Ketones by the Chiral Primary Amine-Catalyzed Asymmetric Retro-Claisen Reaction. Org. Lett. 2022, 24, 1752–1756.
- 49 Xu, C.; Zhang, L.; Luo, S. Asymmetric Enamine Catalysis with β-Ketoesters by Chiral Primary Amine: Divergent Stereocontrol Modes. J. Org. Chem. 2014, 79, 11517–11526
- 50 Chen, W.; Wang, Y.; Mi, X.; Luo, S. Enantioselective Oxidative Coupling of β-Ketocarbonyls and Anilines by Joint Chiral Primary Amine and Selenium Catalysis. Org. Lett. 2019, 21, 8178–8182.
- 51 You, Y.; Zhang, L.; Luo, S. Reagent-Controlled Enantioselectivity Switch for the Asymmetric Fluorination of β-Ketocarbonyls by Chiral Primary Amine Catalysis. Chem. Sci. 2017, 8, 621–626.
- 52 Cui, L.; You, Y.; Mi, X.; Luo, S. Asymmetric Fluorination of α-Branched Aldehydes by Chiral Primary Amine Catalysis: Reagent-Controlled Enantioselectivity Switch. J. Org. Chem. 2018, 83, 4250–4256.
- 53 Cui, L.; You, Y. e.; Mi, X.; Luo, S. Catalytic Enantioselective α-Sulfenylation of β-Ketocarbonyls by Chiral Primary Amines. Org. Chem. Front. 2018, 5, 2313–2316.
- 54 Zhang, Q.; Shi, M.; Mi, X.; Luo, S. Catalytic Asymmetric Oxidative Sulfenylation of β-Ketocarbonyls Using a Chiral Primary Amine. Org. Chem. Front. 2022, 9, 1276–1281.
- 55 Wang, D.; Xu, C.; Zhang, L.; Luo, S. Asymmetric α-Benzoyloxylation of β-Ketocarbonyls by a Chiral Primary Amine Catalyst. Org. Lett. 2015, 17, 576–579.
- 56 Xu, C.; Zhang, L.; Luo, S. Catalytic Asymmetric Oxidative α-C-H N,O-Ketalization of Ketones by Chiral Primary Amine. Org. Lett. 2015, 17, 4392–4395.
- 57 Xue, Z.; Li, Y.; Luo, S. Chiral Primary Amine-Catalyzed Divergent Coupling of α-Substituted Acrylaldehydes with α-Diazoesters. ACS Catal. 2020, 10, 10989–10998.
- 58 Yang, Q.; Zhang, J.; Jia, Z.; Yang, C.; Zhang, L.; Luo, S. Asymmetric 1,3-Dipolar Cycloaddition Reactions of Enones by Primary Amine Catalysis. Asian J. Org. Chem. 2019, 8, 1049–1052.
- 59 Zhu, L.; Zhang, L.; Luo, S. Catalytic Desymmetrizing Dehydrogenation of 4-Substituted Cyclohexanones through Enamine Oxidation. Angew. Chem. Int. Ed. 2018, 57, 2253–2258.
- 60 Zhu, L.; Zhang, L.; Luo, S. Catalytic Asymmetric β-C-H Functionalizations of Ketones via Enamine Oxidation. Org. Lett. 2018, 20, 1672–1675.
- 61 Yang, Q.; Zhang, L.; Ye, C.; Luo, S.; Wu, L.-Z.; Tung, C.-H. Visible-Light-Promoted Asymmetric Cross-Dehydrogenative Coupling of Tertiary Amines to Ketones by Synergistic Multiple Catalysis. Angew. Chem. Int. Ed. 2017, 56, 3694–3698.
- 62 Li, L.; Li, Y.; Fu, N.; Zhang, L.; Luo, S. Catalytic Asymmetric Electrochemical α-Arylation of Cyclic β-Ketocarbonyls with Anodic Benzyne Intermediates. Angew. Chem. Int. Ed. 2020, 59, 14347–14351.
- 63 Wang, Y.; Zhang, J.; You, C.; Mi, X.; Luo, S. Catalytic Asymmetric Addition and Telomerization of Butadiene with Enamine Intermediates. CCS Chem. 2022, 4, 2267–2275.
- 64 Zhang, J.; Wang, Y.; You, C.; Shi, M.; Mi, X.; Luo, S. Asymmetric Coupling of β-Ketocarbonyls and Alkynes by Chiral Primary Amine/Rh Synergistic Catalysis. Org. Lett. 2022, 24, 1186–1189.
- 65 Wang, D.; Zhang, L.; Luo, S. Visible Light Promoted β-C-H Alkylation of β-Ketocarbonyls via a β-Enaminyl Radical Intermediate. Chin. J. Chem. 2018, 36, 311–320.
- 66 Jia, Z.; Zhang, L.; Luo, S. Asymmetric C–H Dehydrogenative Allylic Alkylation by Ternary Photoredox-Cobalt-Chiral Primary Amine Catalysis under Visible Light. J. Am. Chem. Soc. 2022, 144, 10705–10710.
- 67 Fu, N.; Zhang, L.; Luo, S. Catalytic Asymmetric Enamine Protonation Reaction. Org. Biomol. Chem. 2018, 16, 510–520.
- 68 Huang, M.; Zhang, L.; Pan, T.; Luo, S. Deracemization through Photochemical E/Z Isomerization of Enamines. Science 2022, 375, 869–874.
- 69 Franzén, J.; Marigo, M.; Fielenbach, D.; Wabnitz, T. C.; Kjærsgaard, A.; Jørgensen, K. A. A General Organocatalyst for Direct α-Functionalization of Aldehydes: Stereoselective C−C, C−N, C−F, C−Br, and C−S Bond-Forming Reactions. Scope and Mechanistic Insights. J. Am. Chem. Soc. 2005, 127, 18296–18304.
- 70 Hayashi, Y.; Gotoh, H.; Hayashi, T.; Shoji, M. Diphenylprolinol Silyl Ethers as Efficient Organocatalysts for the Asymmetric Michael Reaction of Aldehydes and Nitroalkenes. Angew. Chem. Int. Ed. 2005, 44, 4212–4215.
- 71 Marigo, M.; Wabnitz, T. C.; Fielenbach, D.; Jørgensen, K. A. Enantioselective Organocatalyzed α-Sulfenylation of Aldehydes. Angew. Chem. Int. Ed. 2005, 44, 794–797.
- 72 Wang, Y.; Zhou, H.; Yang, K.; You, C.; Zhang, L.; Luo, S. Steric Effect of Protonated Tertiary Amine in Primary-Tertiary Diamine Catalysis: A Double-Layered Sterimol Model. Org. Lett. 2019, 21, 407–411.
- 73 Zhou, P.; Zhang, L.; Luo, S.; Cheng, J.-P. Asymmetric Synthesis of Wieland-Miescher and Hajos-Parrish Ketones Catalyzed by an Amino-Acid-Derived Chiral Primary Amine. J. Org. Chem. 2012, 77, 2526–2530.
- 74 Xu, C.; Zhang, L.; Zhou, P.; Luo, S.; Cheng, J.-P. A Practical Protocol for Asymmetric Synthesis of Wieland–Miescher and Hajos–Parrish Ketones Catalyzed by a Simple Chiral Primary Amine. Synthesis 2013, 45, 1939–1945.
- 75 Ushakov, D. B.; Raja, A.; Franke, R.; Sasse, F.; Maier, M. E. Total Synthesis of (±)-Moluccanic Acid Methyl Ester. Synlett 2012, 23, 1358–1360.
- 76 Tahara, T.; Streit, U.; Pelish, H. E.; Shair, M. D. STAT3 Inhibitory Activity of Structurally Simplified Withaferin A Analogues. Org. Lett. 2017, 19, 1538–1541.
- 77 Economou, C.; Tomanik, M.; Herzon, S. B. Synthesis of Myrocin G, the Putative Active Form of the Myrocin Antitumor Antibiotics. J. Am. Chem. Soc. 2018, 140, 16058–16061.
- 78 Trzoss, L.; Xu, J.; Lacoske, M. H.; Mobley, W. C.; Theodorakis, E. A. Illicium Sesquiterpenes: Divergent Synthetic Strategy and Neurotrophic Activity Studies. Chem. - Eur. J. 2013, 19, 6398–6408.
- 79 Qiao, T.; Wang, Y.; Zheng, S.; Kang, H.; Liang, G. Total Syntheses of Norrisolide-Type Spongian Diterpenes Cheloviolene C, Seconorrisolide B, and Seconorrisolide C. Angew. Chem. Int. Ed. 2020, 59, 14111–14114.
- 80 Ma, B.; Zhao, Y.; He, C.; Ding, H. Total Synthesis of an Atropisomer of the Schisandra Triterpenoid Schiglautone A. Angew. Chem. Int. Ed. 2018, 57, 15567–15571.
- 81 Zhang, D.-W.; Fan, H.-L.; Zhang, W.; Li, C.-J.; Luo, S.; Qin, H.-B. Collective Enantioselective Total Synthesis of (+)-Sinensilactam A, (+)-Lingzhilactone B and (−)-Lingzhiol: Divergent Reactivity of Styrene. Chem. Commun. 2020, 56, 10066–10069.
- 82 Daumann, L. J. Essential and Ubiquitous: The Emergence of Lanthanide Metallobiochemistry. Angew. Chem. Int. Ed. 2019, 58, 12795–12802.
- 83 Mure, M. Tyrosine-Derived Quinone Cofactors. Acc. Chem. Res. 2004, 37, 131–139.
- 84 Anthony, C. Quinoprotein-Catalysed Reactions. Biochem. J. 1996, 320, 697–711.
- 85 Hauge, J. G. Glucose Dehydrogenase of Bacterium Anitratum: An Enzyme with a Novel Prosthetic Group. J. Biol. Chem. 1964, 239, 3630–3639.
- 86 Anthony, C.; Zatman, L. J. The Microbial Oxidation of Methanol. Purification and Properties of the Alcohol Dehydrogenase of Pseudomonas sp. M27. Biochem. J. 1967, 104, 953–959.
- 87 Klinman, J. P. Mechanisms Whereby Mononuclear Copper Proteins Functionalize Organic Substrates. Chem. Rev. 1996, 96, 2541–2562.
- 88 Janes Susan, M.; Mu, D.; Wemmer, D.; Smith Alan, J.; Kaur, S.; Maltby, D.; Burlingame Alma, L.; Klinman Judith, P. A New Redox Cofactor in Eukaryotic Enzymes: 6-Hydroxydopa at the Active Site of Bovine Serum Amine Oxidase. Science 1990, 248, 981–987.
- 89 Largeron, M.; Fleury, M. B. Oxidative Deamination of Benzylamine by Electrogenerated Quinonoid Systems as Mimics of Amine Oxidoreductases Cofactors. J. Org. Chem. 2000, 65, 8874–8881.
- 90 Wendlandt, A. E.; Stahl, S. S. Chemoselective Organocatalytic Aerobic Oxidation of Primary Amines to Secondary Imines. Org. Lett. 2012, 14, 2850–2853.
- 91 Zhu, X.-Q.; Wang, C.-H.; Liang, H.; Cheng, J.-P. Theoretical Prediction of the Hydride Affinities of Various p- and o-Quinones in DMSO. J. Org. Chem. 2007, 72, 945–956.
- 92 Zhu, X.-Q.; Wang, C.-H. Accurate Estimation of the One-Electron Reduction Potentials of Various Substituted Quinones in DMSO and CH3CN. J. Org. Chem. 2010, 75, 5037–5047.
- 93 Zhu, X.-Q.; Wang, C.-H.; Liang, H. Scales of Oxidation Potentials, pKa, and BDE of Various Hydroquinones and Catechols in DMSO. J. Org. Chem. 2010, 75, 7240–7257.
- 94 Qin, Y.; Zhang, L.; Lv, J.; Luo, S.; Cheng, J.-P. Bioinspired Organocatalytic Aerobic C-H Oxidation of Amines with an ortho-Quinone Catalyst. Org. Lett. 2015, 17, 1469–1472.
- 95 Zhang, R.; Qin, Y.; Zhang, L.; Luo, S. Mechanistic Studies on Bioinspired Aerobic C-H Oxidation of Amines with an ortho-Quinone Catalyst. J. Org. Chem. 2019, 84, 2542–2555.
- 96 Steves, J. E.; Stahl, S. S. Copper(I)/ABNO-Catalyzed Aerobic Alcohol Oxidation: Alleviating Steric and Electronic Constraints of Cu/TEMPO Catalyst Systems. J. Am. Chem. Soc. 2013, 135, 15742–15745.
- 97 Zhang, R.; Zhang, R.; Jian, R.; Zhang, L.; Zhang, M.-T.; Xia, Y.; Luo, S. Bio-Inspired Lanthanum-ortho-Quinone Catalysis for Aerobic Alcohol Oxidation: semi-Quinone Anionic Radical as Redox Ligand. Nat. Commun. 2022, 13, 428–437.
- 98 Frank, J.; Dijkstra, M.; Duine, J. A.; Balny, C. Kinetic and Spectral Studies on the Redox Forms of Methanol Dehydrogenase from Hyphomicrobium X. Eur. J. Biochem. 1988, 174, 331–338.
- 99 Itoh, S.; Ogino, M.; Fukui, Y.; Murao, H.; Komatsu, M.; Ohshiro, Y.; Inoue, T.; Kai, Y.; Kasai, N. C-4 and C-5 Adducts of Cofactor PQQ (Pyrroloquinolinequinone). Model Studies Directed toward the Action of Quinoprotein Methanol Dehydrogenase. J. Am. Chem. Soc. 1993, 115, 9960–9967.
- 100 Idupulapati, N. B.; Mainardi, D. S. Quantum Chemical Modeling of Methanol Oxidation Mechanisms by Methanol Dehydrogenase Enzyme: Effect of Substitution of Calcium by Barium in the Active Site. J. Phys. Chem. A 2010, 114, 1887–1896.
- 101 Kay, C. W. M.; Mennenga, B.; Gorisch, H.; Bittl, R. Substrate Binding in Quinoprotein Ethanol Dehydrogenase from Pseudomonas aeruginosa Studied by Electron-Nuclear Double Resonance. Proc. Natl. Acad. Sci. U. S. A. 2006, 103, 5267–5272.
- 102 Koksal, M.; Jin, Y. H.; Coates, R. M.; Croteau, R.; Christianson, D. W. Taxadiene Synthase Structure and Evolution of Modular Architecture in Terpene Biosynthesis. Nature 2011, 469, 116–120.
- 103 Lv, J.; Li, X.; Zhong, L.; Luo, S.; Cheng, J.-P. Asymmetric Binary-Acid Catalysis with Chiral Phosphoric Acid and MgF2: Catalytic Enantioselective Friedel−Crafts Reactions of β,γ-Unsaturated α-Ketoesters. Org. Lett. 2010, 12, 1096–1099.
- 104 Zhang, L.; Chen, L.; Lv, J.; Cheng, J.-P.; Luo, S. Theoretical Studies of the Asymmetric Binary-Acid-Catalyzed tert-Aminocyclization Reaction: Origins of the C-sp3-H Activation and Stereoselectivity. Chem. Asian J. 2012, 7, 2569–2576.
- 105 Chen, L.; Zhang, L.; Lv, J.; Cheng, J.-P.; Luo, S. Catalytic Enantioselective tert-Aminocyclization by Asymmetric Binary Acid Catalysis (ABC): Stereospecific 1,5-Hydrogen Transfer. Chem. - Eur. J. 2012, 18, 8891–8895.
- 106 Lv, J.; Zhang, L.; Luo, S.; Cheng, J.-P. Switchable Diastereoselectivity in Enantioselective [4+2] Cycloadditions with Simple Olefins by Asymmetric Binary Acid Catalysis. Angew. Chem. Int. Ed. 2013, 52, 9786–9790.
- 107 Lv, J.; Zhang, L.; Hu, S.; Cheng, J.-P.; Luo, S. Asymmetric Binary-Acid Catalysis with InBr3 in the Inverse-Electron-Demanding Hetero-Diels- Alder Reaction of Mono- and Bis-Substituted Cyclopentadienes: Remote Fluoro-Effect on Stereocontrol. Chem. - Eur. J. 2012, 18, 799–803.
- 108 Wang, L.; Lv, J.; Zhang, L.; Luo, S. Catalytic Regio- and Enantioselective [4+2] Annulation Reactions of Non-activated Allenes by a Chiral Cationic Indium Complex. Angew. Chem. Int. Ed. 2017, 56, 10867–10871.
- 109 Zhong, X.; Lv, J.; Luo, S. Enantio- and Diastereoselective Cyclopropanation of β,γ-Unsaturated α-Ketoester by a Chiral Phosphate/Indium(III) Complex. Org. Lett. 2017, 19, 3331–3334.
- 110 Lv, J.; Zhong, X.; Luo, S. Taming Living Carbocations in Catalytic Direct Conjugate Addition of Simple Alkenes to α,β-Enones. Chem. - Eur. J. 2014, 20, 8293–8296.
- 111 Yang, Z.; Li, H.; Zhang, L.; Zhang, M.-T.; Cheng, J.-P.; Luo, S. Organic Photocatalytic Cyclization of Polyenes: A Visible-Light-Mediated Radical Cascade Approach. Chem. - Eur. J. 2015, 21, 14723–14727.
- 112 Yang, Z.; Li, S.; Luo, S. Total Synthesis of (±)-Hongoquercin A via Visible-Light-Mediated Organocatalytic Polyene Cyclization. Acta Chim. Sinica 2017, 75, 351–354.
- 113 Yang, Z.; Li, H.; Li, S.; Zhang, M.-T.; Luo, S. A Chiral Ion-Pair Photoredox Organocatalyst: Enantioselective anti-Markovnikov Hydroetherification of Alkenols. Org. Chem. Front. 2017, 4, 1037–1041.
- 114 Mukaiyama, T.; Kobayashi, S.; Shoda, S.-i. A Facile Synthesis of α-Glucosides and α-Ribosides from the Corresponding 1-O-Acyl Sugars and Alcohols in the Presence of Trityl Perchlorate. Chem. Lett. 1984, 13, 907–910.
- 115 Mukaiyama, T.; Kobayashi, S.; Shoda, S.-i. A Facile Synthesis of α-C-Ribofuranosides from 1-O-Acetyl Ribose in the Presence of Trityl Perchlorate. Chem. Lett. 1984, 13, 1529–1530.
- 116 Mukaiyama, T.; Kobayashi, S.; Murakami, M. Trityl Perchlorate as an Efficient Catalyst in the Aldol-Type Reaction. Chem. Lett. 1984, 1759–1762.
- 117 Ohshima, M.; Murakami, M.; Mukaiyama, T. A Convenient Method for the Prepareation of γ-Ketosulfides from Thioacetals. Chem. Lett. 1985, 14, 1871–1874.
- 118 Mukaiyama, T.; Kobayashi, S.; Murakami, M. An Efficient Method for the Preparation of threo Cross-Aldols from Silyl Enol Ethers and Aldehydes Using Trityl Perchlorate as a Catalyst. Chem. Lett. 1985, 14, 447–450.
- 119 Kobayashi, S.; Murakami, M.; Mukaiyama, T. The Trityl Perchlorate Catalyzed Michael Reaction. Chem. Lett. 1985, 14, 953–956.
- 120 Mukaiyama, T.; Akamatsu, H.; Han, J. S. A Convenient Method for Stereoselective Synthesis of β-Aminoesters. Iron(II) Iodide or Trityl Hexachloroantimonate as an Effective Catalyst in the Reaction of Ketene Silyl Acetals with Imines. Chem. Lett. 1990, 889–892.
- 121 Kobayashi, S.; Murakami, M.; Mukaiyama, T. Trityl Salts as Efficient Catalysts in the Aldol Reaction. Chem. Lett. 1985, 1535–1538.
- 122 Kobayashi, S.; Matsui, S.; Mukaiyama, T. Trityl Salt Catalyzed Aldol Reaction between α,β-Acetylenic Ketones and Silyl Enol Ethers. Chem. Lett. 1988, 17, 1491–1494.
- 123 Bah, J.; Franzen, J. Carbocations as Lewis Acid Catalysts in Diels-Alder and Michael Addition Reactions. Chem. - Eur. J. 2014, 20, 1066–1072.
- 124 Bah, J.; Naidu, V. R.; Teske, J.; Franzen, J. Carbocations as Lewis Acid Catalysts: Reactivity and Scope. Adv. Synth. Catal. 2015, 357, 148–158.
- 125 Naidu, V. R.; Bah, J.; Franzen, J. Direct Organocatalytic Oxo-Metathesis, a trans-Selective Carbocation-Catalyzed Olefination of Aldehydes. Eur. J. Org. Chem. 2015, 2015, 1834–1839.
- 126 El Remaily, M. A. E. A. A. A.; Naidu, V. R.; Ni, S.; Franzén, J. Carbocation Catalysis: Oxa-Diels–Alder Reactions of Unactivated Aldehydes and Simple Dienes. Eur. J. Org. Chem. 2015, 2015, 6610–6614.
- 127 Ni, S.; El Remaily, M. A. E. A. A. A.; Franzén, J. Carbocation Catalyzed Bromination of Alkyl Arenes, a Chemoselective sp3 vs. sp2 C−H Functionalization. Adv. Synth. Catal. 2018, 360, 4197–4204.
- 128 Ni, S. J.; Franzen, J. Carbocation Catalysed Ring Closing Aldehyde- Olefin Metathesis. Chem. Commun. 2018, 54, 12982–12985.
- 129 Riant, O.; Samuel, O.; Kagan, H. B. A General Asymmetric Synthesis of Ferrocenes with Planar Chirality. J. Am. Chem. Soc. 1993, 115, 5835–5836.
- 130 Taudien, S.; Riant, O.; Kagan, H. B. Synthesis of Chiral Carbocations Linked to a Ferrocene Unit. Tetrahedron Lett. 1995, 36, 3513–3516.
- 131 Sammakia, T.; Latham, H. A. On the Use of Ferrocenyl Cations as Chiral Lewis Acids: Evidence for Protic Acid Catalysis. Tetrahedron Lett. 1995, 36, 6867–6870.
- 132 Chen, C.-T.; Chao, S.-D.; Yen, K.-C.; Chen, C.-H.; Chou, I.-C.; Hon, S.-W. Chiral Triarylcarbenium Ions in Asymmetric Mukaiyama Aldol Additions. J. Am. Chem. Soc. 1997, 119, 11341–11342.
- 133
Chen, C.-T.; Chao, S.-D.; Yen, K.-C. Functionalized Triarylcarbenium Ions as Catalysts in Mukaiyama Aldol Addition: Effects of Counter Ions and Silyl Groups on the Intervention of Silyl Catalysis. Synlett 1998, 1998, 924–926.
10.1055/s-1998-1815 Google Scholar
- 134 Lv, J.; Zhang, Q.; Zhong, X.; Luo, S. Asymmetric Latent Carbocation Catalysis with Chiral Trityl Phosphate. J. Am. Chem. Soc. 2015, 137, 15576–15583.
- 135 Zhang, Q.; Lv, J.; Luo, S. Enantioselective Diels–Alder Reaction of Anthracene by Chiral Tritylium Catalysis. Beilstein J. Org. Chem. 2019, 15, 1304–1312.




