Photo-triggered Intramolecular Radical Tandem Regioselective Alkylation/Cyclization of 1,6-Dienes with Redox-Active Esters Enabled by an EDA Complex
Bin Sun
Collaborative Innovation Center of Yangtze River Delta Region Green Pharmaceuticals, Zhejiang University of Technology, Hangzhou, Zhejiang, 310014 China
Search for more papers by this authorLan Ling
College of Pharmaceutical Sciences, Zhejiang University of Technology, Hangzhou, Zhejiang, 310014 China
Search for more papers by this authorXiaohui Zhuang
Collaborative Innovation Center of Yangtze River Delta Region Green Pharmaceuticals, Zhejiang University of Technology, Hangzhou, Zhejiang, 310014 China
Search for more papers by this authorLulu Yang
Collaborative Innovation Center of Yangtze River Delta Region Green Pharmaceuticals, Zhejiang University of Technology, Hangzhou, Zhejiang, 310014 China
Search for more papers by this authorJieli Yin
Collaborative Innovation Center of Yangtze River Delta Region Green Pharmaceuticals, Zhejiang University of Technology, Hangzhou, Zhejiang, 310014 China
Search for more papers by this authorCorresponding Author
Can Jin
Collaborative Innovation Center of Yangtze River Delta Region Green Pharmaceuticals, Zhejiang University of Technology, Hangzhou, Zhejiang, 310014 China
College of Pharmaceutical Sciences, Zhejiang University of Technology, Hangzhou, Zhejiang, 310014 China
E-mail: [email protected]Search for more papers by this authorBin Sun
Collaborative Innovation Center of Yangtze River Delta Region Green Pharmaceuticals, Zhejiang University of Technology, Hangzhou, Zhejiang, 310014 China
Search for more papers by this authorLan Ling
College of Pharmaceutical Sciences, Zhejiang University of Technology, Hangzhou, Zhejiang, 310014 China
Search for more papers by this authorXiaohui Zhuang
Collaborative Innovation Center of Yangtze River Delta Region Green Pharmaceuticals, Zhejiang University of Technology, Hangzhou, Zhejiang, 310014 China
Search for more papers by this authorLulu Yang
Collaborative Innovation Center of Yangtze River Delta Region Green Pharmaceuticals, Zhejiang University of Technology, Hangzhou, Zhejiang, 310014 China
Search for more papers by this authorJieli Yin
Collaborative Innovation Center of Yangtze River Delta Region Green Pharmaceuticals, Zhejiang University of Technology, Hangzhou, Zhejiang, 310014 China
Search for more papers by this authorCorresponding Author
Can Jin
Collaborative Innovation Center of Yangtze River Delta Region Green Pharmaceuticals, Zhejiang University of Technology, Hangzhou, Zhejiang, 310014 China
College of Pharmaceutical Sciences, Zhejiang University of Technology, Hangzhou, Zhejiang, 310014 China
E-mail: [email protected]Search for more papers by this authorComprehensive Summary
A photocatalyst- and metal-free radical tandem alkylation/cyclization between 1,6-dienes and redox-active esters has been developed, affording a series of N-aryl pyrrolidine-2-ones in moderate to good yields. The transformation is driven by the formation of an electron-donor-acceptor (EDA) complex and a subsequent single electron transfer (SET) process. This photocatalyst-free protocol features excellent regioselectivity, mild conditions and broad substrate scope, providing a facile access to 3-alkyl-3,4-dimethyl-1-phenylpyrrolidin-2-one.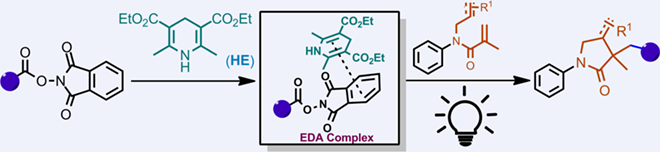
Supporting Information
| Filename | Description |
|---|---|
| cjoc202200516-sup-0001-Supinfo.pdfPDF document, 6 MB |
Appendix S1 Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1(a) Coleman, R. S.; Walczak, M. C.; Campbell, E. L. Total synthesis of Lucilactaene, a cell cycle inhibitor active in p53-inactive cells. J. Am. Chem. Soc. 2005, 127, 16038–16039; (b) Maharjan, S.; Lee, S. B.; Kim, G. J.; Cho, S. J.; Nam, J. -W.; Chin, J.; Choi, H. Isolation of unstable isomers of Lucilactaene and evaluation of anti-inflammatory activity of secondary metabolites produced by the endophytic fungus Fusarium sp. QF001 from the roots of scutellaria baicalenisis. Molecules 2020, 25, 923–935; (c) Abdelhakim, I. A.; Mahmud, F. B.; Motoyama, T.; Futamura, Y.; Takahashi, S.; Osada, H. Dihydrolucilactaene, a potent antimalarial compound from Fusarium sp. RK97-94. J. Nat. Prod. 2022, 85, 63–69.
- 2(a) Shiraki, R.; Sumino, A.; Tadano, K.; Ogawa, S. Total synthesis of natural PI-091, a new platelet aggregation inhibitor of microbial origin. J. Org. Chem. 1996, 61, 2845–2852; (b) Takahashi, S.; Uchida, K.; Nakagawa, A. Biosynthesis of Lactacystin. J. Antibiot. 1995, 48, 1015–1020; (c) Giannopoulos, V.; Tyrikos-Ergas, T.; Myrtollari, K.; Smonou, I. Novel enzymatic reduction of α-amido- and α-cyanoalkyl- β-keto esters catalyzed by ketoreductases. Mol. Catal. 2020, 490, 110952–110957; (d) Omura, S.; Fujimoto, T.; Otoguro, K.; Matsuzaki, K.; Moriguchi, R.; Tanaka, H.; Sasaki, Y. Lactacystin, a novel microbial metabolite, induces neuritogenesis of neuroblastoma cells. J. Antibiot. 1991, 44, 113–116.
- 3 Clarke, B.; Demont, E.; Dingwall, C.; Dunsdon, R.; Faller, A.; Hawkins, J.; Hussain, I.; MacPherson, D.; Maile, G.; Matico, R.; Milner, P.; Mosley, J.; Naylor, A.; O'Brien, A.; Redshaw, S.; Riddell, D.; Rowland, P.; Soleil, V.; Smith, K. J.; Stanway, S.; Stemp, G.; Sweitzer, S.; Theobald, P.; Vesey, D.; Walter, D. S.; Ward, J.; Wayne, G. BACE-1 inhibitors part1: Identification of novel hydroxy ethylamines (HEAs). Bioorg. Med. Chem. Lett. 2008, 18, 1011–1016.
- 4(a) Gein, V. L.; Silina, T. A.; Cherepanov, A. A.; Shishkin, A. P.; Bulatov, I. P.; Dozmorova, N. V.; Makhmudov, R. R. Synthesis and biological activity of 1-substituted 5-oxopyrrolidine-3-carboxylic acids. Pharm. Chem. J. 2021, 55, 23–25; (b) Chang, K.; Shi, Y.; Chen, J.; He, Z.; Xu, Z.; Zhao, Z.; Zhu, W.; Li, H.; Xu, Y.; Li, B. J.; Qian, X. The discovery of new plant activators and scaffolds with potential induced systemic resistance: from jasmonic acid to pyrrolidone. Med. Chem. Commun. 2016, 7, 1849–1857; (c) Sun, J.-Q.; Jiang, H.-L.; Li, C.-Y. Systemin/jasmonate-mediated systemic defense signaling in tomato. Mol. Plant 2011, 4, 607–615.
- 5(a) Bebbington, A.; Brimblecombe, R. W.; Shakeshaft, D. The central and peripheral activity of acetylenic amines related to oxotremorine. Brit. J. Pharmacol. 1966, 26, 56–57; (b) Kundu, D.; Bhadra, S.; Mukherjee, N.; Sreedhar, B.; Ranu, B. C. Heterogeneous CuII-catalysed solvent-controlled selective N-arylation of cyclic amides and amines with bromo-iodoarenes. Chem. - Eur. J. 2013, 19, 15759–15768.
- 6(a) Wang, T.; Xu, H.; He, J.; Zhang, Y. Investigation towards the reductive amination of levulinic acid by B(C6F5)3/hydrosilane system. Tetrahedron 2020, 76, 131394–131402; (b) Vorobyeva, E.; Gerken, V. C.; Mitchell, S.; Sabadell-Rendón, A.; Hauert, R.; Xi, S.; Borgna, A.; Klose, D.; Collins, S. M.; Midgley, P. A.; Kepaptsoglou, D. M.; Ramasse, Q. M.; Ruiz-Ferrando, A.; Fako, E.; Ortuño, M. A.; López, N.; Carreira, E. M.; Pérez-Ramírez, J. Activation of copper species on carbon nitride for enhanced activity in the arylation of amines. ACS Catal. 2020, 10, 11069–11080; (c) Zhao, H.; Leonori, D. Minimization of back-electron transfer enables the elusive sp3 C-H functionalization of secondary anilines. Angew. Chem. Int. Ed. 2021, 60, 7669-7674; (d) Joensuu, P. M.; Murray, G. J.; Fordyce, E. A. F.; Luebbers T.; Lam, H. W. Diastereoselective nickel-catalyzed reductive aldol cyclizations using diethylzinc as the stoichiometric reductant: scope and mechanistic insight. J. Am. Chem. Soc. 2008, 130, 7328–7338.
- 7(a) Zheng, Y.; Nie, X.; Long, Y.; Ji, L.; Fu, H.; Zheng, X.; Chen, H.; Li, R. Ruthenium-catalyzed synthesis of N-substituted lactams by acceptorless dehydrogenative coupling of diols with primary amines. Chem. Commun. 2019, 55, 12384–12387; (b) Waly, M. A.; Yossif, S. A.; Ibrahim, I. T.; Sofan, M. A. Efficient synthesis of N-substituted 2,4-azepandione ring system as an active intermediate for heterocyclic syntheses. J. Heterocyclic Chem. 2017, 54, 1318–1326; (c) Luo, Y.; Hu, M.; Ge, J.; Li, B.; He, L. Rh-catalyzed oxidation and trifluoroethoxylation of N-aryl-pyrrolidin-2-ones: a domino approach for the synthesis of N-aryl-5-(2, 2, 2-trifluoroethoxy)-1,5-dihydro-2H-pyrrol- 2-ones. Org. Chem. Front. 2022, 9, 1593–1598.
- 8(a) Liu, Y.; Zhang, J. -L.; Song, R.-J.; Li, J.-H. Visible-light-facilitated 5-exo-trig cyclization of 1,6-dienes with alkyl chlorides: selective scission of the C(sp3)-H bond in alkyl chlorides. Eur. J. Org. Chem. 2014, 2014, 1177–1181; (b) Huang, X.-J.; Qin, F.-H.; Liu, Y.; Wu, S.-P.; Li, Q.; Wei, W.-T. Acylation/cyclization of 1,6-dienes with ethers under catalyst- and base-free conditions. Green Chem. 2020, 22, 3952–3955.
- 9(a) Huang, B.; Li, Y.; Yang, C.; Xia, W. Three-component aminoselenation of alkenes via visible-light enabled Fe-catalysis. Green Chem. 2020, 22, 2804–2809; (b) Immel, J. R.; Bloom, S. carba-nucleopeptides (cNPs): a biopharmaceutical modality formed through aqueous Rhodamine B photoredox catalysis. Angew. Chem. Int. Ed. 2022, 61, e202205606; (c) Zhou, Q.-Q.; Zou, Y.-Q.; Lu, L.-Q.; Xiao, W.-J. Visible-light-induced organic photochemical reactions through energy-transfer pathways. Angew. Chem. Int. Ed. 2019, 58, 1586–1604; (d) Cybularczyk-Cecotka, M.; Predygier, J.; Crespi, S.; Szczepanik, J.; Giedyk, M. Photocatalysis in aqueous micellar media enables divergent C-H arylation and N-dealkylation of benzamides. ACS Catal. 2022, 12, 3543–3549; (e) Wang, P.-Z.; Chen, J.-R.; Xiao, W.-J. Hantzsch esters: an emerging versatile class of reagents in photoredox catalyzed organic synthesis. Org. Biomol. Chem. 2019, 17, 6936–6951; (f) Wei, Y.; Zhou, Q.-Q.; Tan, F.; Lu, L.-Q.; Xiao, W. J. Visible-light-driven organic photochemical reactions in the absence of external photocatalysts. Synthesis 2019, 51, 3021–3054.
- 10(a) Lin, L.; Yang, Z.; Liu, J.; Wang, J.; Zheng, J.; Li, J.-L.; Zhang, X.; Liu, X.-W.; Jiang, H.; Li, J. Visible-light-induced surfactant-promoted sulfonylation of alkenes and alkynes with sulfonyl chloride by the formation of an EDA-complex with NaI in water at room temperature. Green Chem. 2021, 23, 5467–5473; (b) Zhen, J.; Du, X.; Xu, X.; Li, Y.; Yuan, H.; Xu, D.; Xue, C.; Luo, Y. Visible-light-mediated late-stage sulfonylation of boronic acids via N-S bond activation of sulfonamides. ACS Catal. 2022, 12, 1986–1991; (c) Cabrera-Afonso, M. J.; Granados, A.; Molander, G. A. Sustainable thioetherification via electron donor-acceptor photoactivation using thianthrenium salts. Angew. Chem. Int. Ed. 2022, 61, e202202706; (d) Wang, H.; Wu, J.; Noble, A.; Aggarwal, V. K. Selective coupling of 1,2-bis-boronic esters at the more substituted site through visible-light activation of electron donor-acceptor complexes. Angew. Chem. Int. Ed. 2022, 61, e202202061; (e) Zhang, J.; Li, Y.; Xu, R.; Chen, Y. Donor-acceptor complex enables alkoxyl radical generation for metal-free C(sp3)-C(sp3) cleavage and allylation/alkenylation. Angew. Chem. Int. Ed. 2017, 56, 12619–12623; (f) Li, Y.; Zhang, J.; Li, D.; Chen, Y. Metal-free C(sp3)-H allylation via aryl carboxyl radicals enabled by donor-acceptor complex. Org. Lett. 2018, 20, 3296–3299; (g) Lima, C. G. S.; Lima, T. M.; Duarte, M.; Jurberg, I. D.; Paixão, M. W. Organic synthesis enabled by light-irradiation of of EDA complexes: theoretical background and synthetic applications. ACS Catal. 2016, 6, 1389–1407; (h) Crisenza, G. E. M.; Mazzarella, D.; Melchiorre, P. Synthetic methods driven by the photoactivity of electron donor-acceptor complexes. J. Am. Chem. Soc. 2020, 142, 5461–5476.
- 11(a) Sun, B.; Shi, X.; Zhuang, X.; Huang, P.; Shi, R.; Zhu, R.; Jin, C. Photoinduced EDA complexes enabled radical tandem cyclization/arylation of unactivated alkene with 2-amino-1,4-naphthoquinones. Org. Lett. 2021, 23, 1862–1867; (b) Yang, J.; Sun, B.; Ding, H.; Huang, P.-Y.; Tang, X.-L.; Shi, R.-C.; Yan, Z.-Y.; Yu, C.-M.; Jin, C. Photo-triggered self-catalyzed fluoroalkylation/cyclization of unactivated alkenes: synthesis of quinazolinones containing the CF2R group. Green Chem. 2021, 23, 575–581; (c) Zhuang, X.; Shi, X.; Zhu, R.; Sun, B.; Su, W.; Jin, C. Photocatalytic intramolecular radical cyclization involved synergistic SET and HAT: synthesis of 3,3-difluoro-γ-lactams. Org. Chem. Front. 2021, 8, 736–742.
- 12(a) Renny, J. S.; Tomasevich, L. L.; Tallmadge, E. H.; Collum, D. B. Method of continuous variations: applications of Job plots to study of molecular associations in organometallic chemistry. Angew. Chem. Int. Ed. 2013, 52, 11998–12013; (b) Li, J.; Tan, S. S.; Kyne, S. H.; Chan, P. W. H. Minisci-type alkylation of N-heteroarenes by N-(acyloxy)phthalimide esters mediated by a Hantzsch ester and blue LED light. Adv. Synth. Catal. 2022, 364, 802–810.
- 13(a) Kuntz, Jr. I. D.; Gasparro, F. P.; Johnston, Jr. M. D.; Taylor, R. P. Molecular interactions and the Benesi-Hildebrand equation. J. Am. Chem. Soc. 1968, 90, 4778–4781; (b) Benesi, H. A.; Hildebrand, J. H. A spectrophotometric investigation of the interaction of iodine with aromatic hydrocarbons. J. Am. Chem. Soc. 1949, 71, 2703–2707.
- 14(a) Chen, Z.; Xue, F.; Liu, T.; Wang, B.; Zhang, Y.; Jin, W.; Xia, Y.; Liu, C. Synthesis of β-hydroxysulfides via visible-light-driven and EDA complex-promoted hydroxysulfenylation of styrenes with heterocyclic thiols in EtOH under photocatalyst-free conditions. Green Chem. 2022, 24, 3250–3256; (b) Correia, J. T. M.; Silva, G. P.; Kisukuri, C. M.; André, E.; Pires, B.; Carneiro, P. S.; Paixão, M. W. Metal-free photoinduced hydroalkylation cascade enabled by an electron-donor- acceptor complex. J. Org. Chem. 2020, 85, 9820–9834.




