Self-assembly at Liquid-Liquid Interface: A New SERS Substrate for Analytical Sensing†
Yue Zhao
Shanghai Key Laboratory of Green Chemistry and Chemical Processes, School of Chemistry and Molecular Engineering, East China Normal University, Dongchuan Road 500, Shanghai, 200241 China
Search for more papers by this authorLu Shi
Shanghai Key Laboratory of Green Chemistry and Chemical Processes, School of Chemistry and Molecular Engineering, East China Normal University, Dongchuan Road 500, Shanghai, 200241 China
Search for more papers by this authorCorresponding Author
Yang Tian
Shanghai Key Laboratory of Green Chemistry and Chemical Processes, School of Chemistry and Molecular Engineering, East China Normal University, Dongchuan Road 500, Shanghai, 200241 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Limin Zhang
Shanghai Key Laboratory of Green Chemistry and Chemical Processes, School of Chemistry and Molecular Engineering, East China Normal University, Dongchuan Road 500, Shanghai, 200241 China
E-mail: [email protected]; [email protected]Search for more papers by this authorYue Zhao
Shanghai Key Laboratory of Green Chemistry and Chemical Processes, School of Chemistry and Molecular Engineering, East China Normal University, Dongchuan Road 500, Shanghai, 200241 China
Search for more papers by this authorLu Shi
Shanghai Key Laboratory of Green Chemistry and Chemical Processes, School of Chemistry and Molecular Engineering, East China Normal University, Dongchuan Road 500, Shanghai, 200241 China
Search for more papers by this authorCorresponding Author
Yang Tian
Shanghai Key Laboratory of Green Chemistry and Chemical Processes, School of Chemistry and Molecular Engineering, East China Normal University, Dongchuan Road 500, Shanghai, 200241 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Limin Zhang
Shanghai Key Laboratory of Green Chemistry and Chemical Processes, School of Chemistry and Molecular Engineering, East China Normal University, Dongchuan Road 500, Shanghai, 200241 China
E-mail: [email protected]; [email protected]Search for more papers by this author†Dedicated to the 120th Anniversary of Northwest Normal University.
Comprehensive Summary
As a highly powerful and sensitive tool, surface enhanced Raman scattering (SERS) has attracted extensive attention in quantification analysis. However, the strong dependence of SERS signal on the detailed local nanostructure makes quantitative SERS analysis suffer from difficulties in controlling the uniformity of nanoscale hot spots and the inefficiency of placing the targeted molecules in prefabricated hot spots. Thus, the development of uniform SERS substrates is becoming an urgent demand to impulse SERS technique for the application in practical systems. The self-assembly of nanoparticles (NPs) at the liquid-liquid interface (LLI) provides a molecular sharp and defect-free focal plane, in which the stable controlling of nanogap sizes and the feasible location of analytes can be virtually guaranteed, leading to greatly enhanced sensitivity and reproducibility of detections. On the other hand, the benefit of liquid/liquid systems allows for either hydrophilic, hydrophobic molecules to be captured and detected individually or simultaneously. Based on these advantages of the new SERS substrate, a variety of self-assembled NP arrays at the LLI have been developed for multiphase analyte detection. Herein, we review recently developed strategies to induce NPs self-assembly at the LLI and discuss the latest research progress on sensitive and reliable SERS analysis in practical applications. Finally, some perspectives are highlighted in the further development of the efficient methods to enhance the plasmonic properties and more practical SERS sensors for practical applications.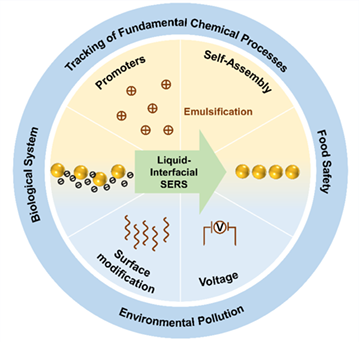
References
- 1 Phan-Quang, G. C.; Lee, H. K.; Ling, X. Y. Isolating Reactions at the Picoliter Scale: Parallel Control of Reaction Kinetics at the Liquid–Liquid Interface. Angew. Chem. Int. Ed. 2016, 128, 8304–8308.
- 2 Cecchini, M. P.; Turek, V. A.; Demetriadou, A.; Britovsek, G.; Welton, T.; Kornyshev, A. A.; Wilton-Ely, JD. E. T.; Edel, J. B. Heavy Metal Sensing Using Self-Assembled Nanoparticles at a Liquid–Liquid Interface. Adv. Opt. Mater. 2014, 2, 966–977.
- 3 Zhang, C.; Liu, Z.; Zhang, L.; Zhu, A.; Liao, F.; Wan, J.; Zhou, J.; Tian, Y. A Robust Au−C≡C Functionalized Surface: Toward Real-Time Mapping and Accurate Quantification of Fe2+ in the Brains of Live AD Mouse Models. Angew. Chem. Int. Ed. 2020, 59, 20499–20507.
- 4 Zhang, L.; Tian, Y. Designing Recognition Molecules and Tailoring Functional Surfaces for In Vivo Monitoring of Small Molecules in the Brain. Acc. Chem. Res. 2018, 51, 688–696.
- 5 Xiao, T.; Wu, F.; Shen, M. Y.; Zhang, M.; Yu, H. H.; Mao, L. Ultrathin Cell-Membrane-Mimic Phosphorylcholine Polymer Film Coating Enables Large Improvements for In Vivo Electrochemical Detection. Angew. Chem. Int. Ed. 2017, 56, 11802–11806.
- 6 Cecchini, M. P.; Turek, V. A.; Paget, J.; Kornyshev, A. A.; Edel, J. B. Self-Assembled Nanoparticle Arrays for Multiphase Trace Analyte Detection. Nat. Mater. 2013, 12, 165–171.
- 7 Li, W.; Jin, J.; Xiong, T.; Yu, P.; Mao, L. Fast-Scanning Potential-Gated Organic Electrochemical Transistors for Highly Sensitive Sensing of Dopamine in Living Rat Brain. Angew. Chem. Int. Ed. 2022, 61, e202204134.
- 8 Liu, Y.; Liu, Z.; Zhao, F.; Tian, Y. Long-Term Tracking and Dynamically Quantifying of Reversible Changes of Extracellular Ca2+ in Multiple Brain Regions of Freely Moving Animals. Angew. Chem. Int. Ed. 2021, 60, 14429–14437.
- 9 Yue, Q.; Li, X.; Wu, F.; Ji, W.; Zhang, Y.; Yu, P.; Zhang, M.; Ma, W.; Wang, M.; Mao, L. Unveiling the Role of DJ-1 Protein in Vesicular Storage and Release of Catecholamine with Nano/Micro-Tip Electrodes. Angew. Chem. Int. Ed. 2020, 59, 11061–11065.
- 10 Shi, L.; Cao, F.; Zhang, L.; Tian, Y. I-motif Formed at Physiological pH Triggered by Spatial Confinement of Nanochannels: An Electrochemical Platform for pH Monitoring in Brain Microdialysates. Anal. Chem. 2020, 92, 4535–4540.
- 11 Dong, H.; Zhou, Q.; Zhang, L.; Tian, Y. Rational Design of Specific Recognition Molecules for Simultaneously Monitoring of Endogenous Polysulfide and Hydrogen Sulfide in the Mouse Brain. Angew. Chem. Int. Ed. 2019, 58, 13948–13953.
- 12 Liu, W.; Dong, H.; Zhang, L.; Tian, Y. Development of an Efficient Biosensor for the in vivo Monitoring of Cu+ and pH in the Brain: Rational Design and Synthesis of Recognition Molecules. Angew. Chem. Int. Ed. 2017, 56, 16328–16332.
- 13 Chen, C.; Pan, Y.; Li, D.; Han, Y.; Zhang, Q. W.; Tian, Y. An Intramolecular Charge Transfer-Forster Resonance Energy Transfer Integrated Unimolecular Platform for Two-Photon Ratiometric Fluorescence Sensing of Methionine Sulfoxide Reductases in Live-Neurons and Mouse Brain Tissues. Anal. Chem. 2022, 94, 6289–6296.
- 14 Liu, Z.; Tian, Y. Recent Advances in Development of Devices and Probes for Sensing and Imaging in the Brain. Sci. China Chem. 2021, 64, 915–931.
- 15 Wu, Z.; Liu, M.; Liu, Z.; Tian, Y. Real-Time Imaging and Simultaneous Quantification of Mitochondrial H2O2 and ATP in Neurons with a Single Two-Photon Fluorescence-Lifetime-Based Probe. J. Am. Chem. Soc. 2020, 142, 7532–7541.
- 16 Liu, Z.; Pei, H.; Zhang, L.; Tian, Y. Mitochondria-Targeted DNA Nanoprobe for Real-Time Imaging and Simultaneous Quantification of Ca2+ and pH in Neurons. ACS Nano 2018, 12, 12357–12368.
- 17 Gong, Z.; Liu, Z.; Zhang, Z.; Mei, Y.; Tian, Y. A Highly Stable Two-photon Ratiometric Fluorescence Probe for Real-time Biosensing and Imaging of Nitric Oxide in Brain Tissues and Larval Zebrafish. CCS Chem. 2022, 4, 2020–2030.
- 18 Liu, Z.; Zhang, Z.; Liu, Y.; Mei, Y.; Feng, E.; Liu, Y.; Zheng, T.; Chen, J.; Zhang, S.; Tian, Y. Raman Fiber Photometry for Understanding Mitochondrial Superoxide Burst and Extracellular Calcium Ion Influx upon Acute Hypoxia in the Brain of Freely Moving Animals. Angew. Chem. Int. Ed. 2022, 61, e202111630.
- 19 Zhou, Y.; Liu, J.; Zheng, T.; Tian, Y. Label-Free SERS Strategy for in situ Monitoring and Real-Time Imaging of Abeta Aggregation Process in Live Neurons and Brain Tissues. Anal. Chem. 2020, 92, 5910–5920.
- 20 Yang, Z.; Liu, T.; Wang, W.; Zhang, L. Stacked Hexagonal Prism of Ag@Ni-MOF-1 as Functionalized SERS Platform through Rational Integration of Catalytic Synthesis of Dopamine-Quinone at Physiological PH with a Biomimetic Route. Chem. Commun. 2020, 56, 3065–3068.
- 21 Wang, W.; Zhao, F.; Li, M.; Zhang, C.; Shao, Y.; Tian, Y. A SERS Optophysiological Probe for the Real-Time Mapping and Simultaneous Determination of the Carbonate Concentration and pH Value in a Live Mouse Brain. Angew. Chem. Int. Ed. 2019, 58, 5256–5260.
- 22 Dong, H.; Yao, D.; Zhou, Q.; Zhang, L.; Tian, Y. An Integrated Platform for the Capture of Circulating Tumor Cells and in situ SERS Profiling of Membrane Proteins through Rational Spatial Organization of Multi-Functional Cyclic RGD Nanopatterns. Chem. Commun. 2019, 55, 1730–1733.
- 23 Xu, Q.; Liu, W.; Li, L.; Zhou, F.; Zhou, J.; Tian, Y. Ratiometric SERS Imaging and Selective Biosensing of Nitric Oxide in Live Cells Based on Trisoctahedral Gold Nanostructures. Chem. Commun. 2017, 53, 1880–1883.
- 24 Nie, S.; Emory, S. R. Probing Single Molecules and Single Nanoparticles by Surface-Enhanced Raman Scattering. Science 1997, 275, 1102–1106.
- 25 Kneipp, K.; Wang, Y.; Kneipp, H.; Perelman, L. T.; Itzkan, I.; Dasari, R. R.; Feld, M. S. Single Molecule Detection Using Surface-Enhanced Raman Scattering (SERS). Phys. Rev. Lett. 1997, 78, 1667−1670.
- 26 Anker, J. N.; Hall, W. P.; Lyandres, O.; Shah, N. C.; Zhao, J.; Van Duyne, R. P. Biosensing with Plasmonic Nanosensors. Nat. Mater. 2008, 7, 442–453.
- 27 Fleischmann, M.; Hendra, P. J.; McQuillan, A. J. Raman Spectra of Pyridine Adsorbed at a Silver Electrode. Chem. Phys. Lett. 1974, 26, 163−166.
- 28 Jeanmaire, D. L.; Van Duyne, R. P. Surface Raman Spectroelectrochemistry: Part I. Heterocyclic, Aromatic, and Aliphatic Amines Adsorbed on the Anodized Silver Electrode. J. Electroanal. Chem. Interf. Electrochem. 1977, 84, 1−20.
- 29 Albrecht, M. G.; Creighton, J. A. Anomalously Intense Raman Spectra of Pyridine at a Silver Electrode. J. Am. Chem. Soc. 1977, 99, 5215−5217.
- 30 Moskovitsm, M. Surface Roughness and the Enhanced Intensity of Raman Scattering by Molecules Adsorbed on Metals. J. Chem. Phys. 1978, 69, 4159−4161.
- 31 Sharma, B.; Frontiera, R. R.; Henry, A. I.; Ringe, E.; Van Duyne, R. P. SERS: Materials, Applications, and the Future. Mater. Today 2012, 15, 16−25.
- 32 Ding, S. Y.; You, E. M.; Tian, Z. Q.; Moskovits, M. Electromagnetic Theories of Surface-Enhanced Raman Spectroscopy. Chem. Soc. Rev. 2017, 46, 4042−4076.
- 33 Wang, H.; Levin, C. S.; Halas, N. J. Nanosphere Arrays with Controlled Sub-10-nm Gaps as Surface-Enhanced Raman Spectroscopy Substrates. J. Am. Chem. Soc. 2005, 127, 14992–14993.
- 34 Xie, J.; Zhang, Q.; Lee, J. Y.; Wang, D. I. The Synthesis of SERS-Active Gold Nanoflower Tags for in vivo Applications. ACS Nano 2008, 2, 2473−2480.
- 35 Khoury, C. G.; Vo-Dinh, T. Gold Nanostars for Surface-Enhanced Raman Scattering: Synthesis, Characterization and Optimization. J. Phys. Chem. C 2008, 112, 18849−18859.
- 36 Zijlstra, P.; Paulo, P. M.; Orrit, M. Optical Detection of Single Non-Absorbing Molecules Using the Surface Plasmon Resonance of a Gold Nanorod. Nat. Nanotechnol. 2012, 7, 379−382.
- 37 Liu, J.; Liu, Z.; Wang, W.; Tian, Y. Real-time Tracking and Sensing of Cu+ and Cu2+ with a Single SERS Probe in the Live Brain: Toward Understanding Why Copper Ions Were Increased upon Ischemia. Angew. Chem. Int. Ed. 2021, 60, 21351−21359.
- 38 Li, J. F.; Huang, Y. F.; Ding, Y.; Yang, Z. L.; Li, S. B.; Zhou, X. S.; Fan, F. R.; Zhang, W.; Zhou, Z. Y.; Wu, D. Y.; Ren, B.; Wang, Z. L.; Tian, Z. Q. Shell-Isolated Nanoparticle-Enhanced Raman Spectroscopy. Nature 2010, 464, 392−395.
- 39 Gandra, N.; Abbas, A.; Tian, L.; Singamaneni, S. Plasmonic Planet–Satellite Analogues: Hierarchical Self-Assembly of Gold Nanostructures. Nano Lett. 2012, 12, 2645−2651.
- 40 Feng, E.; Tian, Y. Surface-enhanced Raman Scattering of Self-Assembled Superstructures. Chem. Res. Chin. U. 2021, 37, 989−1007.
- 41 Zhou, Y.; Gu, Q.; Qiu, T.; He, X.; Chen, J.; Qi, R.; Huang, R.; Zheng, T.; Tian, Y. Ultrasensitive Sensing of Volatile Organic Compounds Using a Cu-Doped SnO2-NiO p-n Heterostructure That Shows Significant Raman Enhancement. Angew. Chem. Int. Ed. 2021, 60, 26260−26267.
- 42 Zheng, T.; Zhou, Y.; Feng, E.; Tian, Y. Surface-Enhanced Raman Scattering on 2D Nanomaterials: Recent Developments and Applications. Chin. J. Chem. 2021, 39, 745−756.
- 43 Feng, E.; Zheng, T.; He, X.; Chen, J.; Tian, Y. A Novel Ternary Hetero-Structure with Dramatic SERS Activity for Evaluation of PD-L1 Expression at the Single-Cell Level. Sci. Adv. 2018, 4, eaau3494.
- 44 Lu, X.; Wang, H.; He, Y. Controllable Synthesis of Silicon-Based Nanohybrids for Reliable Surface-Enhanced Raman Scattering Sensing. Chin. J. Chem. 2022, 40, 734−745.
- 45 Zong, C.; Xu, M.; Xu, L. J.; Wei, T.; Ma, X.; Zheng, X. S.; Hu, R.; Ren, B. Surface-Enhanced Raman Spectroscopy for Bioanalysis: Reliability and Challenges. Chem. Rev. 2018, 118, 4946−4980.
- 46 Lorén, A.; Engelbrektsson, J.; Eliasson, C.; Josefson, M.; Abrahamsson, J.; Johansson, M.; Abrahamsson, K. Internal Standard in Surface-Enhanced Raman Spectroscopy. Anal. Chem. 2004, 76, 7391−7395.
- 47 Zhang, D.; Xie, Y.; Deb, S. K.; Davison, V. J.; Ben-Amotz, D. Isotope Edited Internal Standard Method for Quantitative Surface-Enhanced Raman Spectroscopy. Anal. Chem. 2005, 77, 3563−3569.
- 48 Shen, W.; Lin, X.; Jiang, C.; Li, C.; Lin, H.; Huang, J.; Wang, S.; Liu, G.; Yan, X.; Zhong, Q. Reliable Quantitative SERS Analysis Facilitated by Core−Shell Nanoparticles with Embedded Internal Standards. Angew. Chem. Int. Ed. 2015, 54, 7308−7312.
- 49 Tian, L.; Su, M.; Yu, F.; Xu, Y.; Li, X.; Li, L.; Liu, H.; Tan, W. Liquid-state Quantitative SERS Analyzer on Self-Ordered Metal Liquid-like Plasmonic Arrays. Nat. Commun. 2018, 9, 3642−3653.
- 50 Quilis, N. G.; Lequeux, M.; Venugopalan, P.; Khan, I.; Knoll, W.; Boujday, S.; Chapelle, M. L.; Dostalek, J. Tunable Laser Interference Lithography Preparation of Plasmonic Nanoparticle Arrays Tailored for SERS. Nanoscale 2018, 10, 10268−10276.
- 51 Chen, J.; Liu, G.; Zhu, Y. Z.; Su, M.; Yin, P.; Wu, X. J.; Lu, Q.; Tan, C.; Zhao, M.; Liu, Z.; Yang, W.; Li, H.; Nam, G. H.; Zhang, L.; Chen, Z.; Huang, X.; Radjenovic, P. M.; Huang, W.; Tian, Z. Q.; Li, J. F.; Zhang, H. Ag@MoS2 Core-Shell Heterostructure as SERS Platform to Reveal the Hydrogen Evolution Active Sites of Single-Layer MoS2. J. Am. Chem. Soc. 2020, 142, 7161−7167.
- 52 Vignolini, S.; Yufa, N. A.; Cunha, P. S.; Guldin, S.; Rushkin, I.; Stefik, M.; Hur, K.; Wiesner, U.; Baumberg, J. J.; Steiner, U. A 3D Optical Metamaterial Made by Self-Assembly. Adv. Mater. 2012, 24, 23−27.
- 53 Song, L.; Qiu, N.; Huang, Y.; Cheng, Q.; Yang, Y.; Lin, H.; Su, F.; Chen, T. Macroscopic Orientational Gold Nanorods Monolayer Film with Excellent Photothermal Anticounterfeiting Performance. Adv. Opt. Mater. 2020, 8, 1902082.
- 54 Ye, Z.; Li, C.; Chen, Q.; Xu, Y.; Bell, S. E. Self-assembly of Colloidal Nanoparticles into 2D Arrays at Water-Oil Interfaces: Rational Con-struction of Stable SERS Substrates with Accessible Enhancing Surfaces and Tailored Plasmonic Response. Nanoscale 2021, 13, 5937−5953.
- 55 Booth, S. G.; Dryfe, R. A. Assembly of Nanoscale Objects at the Liquid/Liquid Interface. J. Phys. Chem. C 2015, 119, 23295−23309.
- 56 Hu, L.; Chen, M.; Fang, X.; Wu, L. Oil–Water Interfacial Self-assembly: a Novel Strategy for Nanofilm and Nanodevice Fabrication. Chem. Soc. Rev. 2012, 41, 1350−1362.
- 57 Scanlon, M. D.; Smirnov, E.; Stockmann, T. J.; Peljo, P. Gold Nanofilms at Liquid-Liquid Interfaces: An Emerging Platform for Redox Electrocatalysis, Nanoplasmonic Sensors, and Electrovariable Optics. Chem. Rev. 2018, 118, 3722−3751.
- 58 Reincke, F.; Hickey, S. G.; Kegel, W. K.; Vanmaekelbergh, D. Spontaneous Assembly of a Monolayer of Charged Gold Nanocrystals at the Water/Oil Interface. Angew. Chem. Int. Ed. 2004, 43, 458−462.
- 59 Duan, H.; Wang, D.; Kurth, D. G.; Möhwald, H. Directing Self-assembly of Nanoparticles at Water/Oil Interfaces. Angew. Chem. Int. Ed. 2004, 43, 5639−5642.
- 60 Tian, H.; Li, H.; Fang, Y. Binary Thiol-Capped Gold Nanoparticle Monolayer Films for Quantitative Surface-Enhanced Raman Scattering Analysis. ACS Appl. Mater. Interfaces 2019, 11, 16207−16213.
- 61 Lu, X.; Huang, Y.; Liu, B.; Zhang, L.; Song, L.; Zhang, J.; Zhang, A.; Chen, T. Light-Controlled Shrinkage of Large-Area Gold Nanoparticle Monolayer Film for Tunable SERS Activity. Chem. Mater. 2018, 30, 1989−1997.
- 62 Ding, T.; Rudrum, A. W.; Herrmann, L. O.; Turke, V.; Baumberg, J. J. Polymer-Assisted Self-Assembly of Gold Nanoparticle Monolayers and Their Dynamical Switching. Nanoscale 2016, 8, 15864−15869.
- 63 Park, Y. K.; Park, S. Directing Close-Packing of Midnanosized Gold Nanoparticles at a Water/Hexane Interface. Chem. Mater. 2008, 20, 2388−2393.
- 64 Park, Y. K.; Yoo, S. H.; Park, S. Assembly of Highly Ordered Nanoparticle Monolayers at a Water/Hexane Interface. Langmuir 2007, 23, 10505−10510.
- 65 Li, C.; Xu, Y.; Li, X.; Ye, Z.; Yao, C.; Chen, Q.; Zhang, Y.; Bell, S. E. J. Unexpected Dual Action of Cetyltrimethylammonium Bromide (CTAB) in the Self-Assembly of Colloidal Nanoparticles at Liquid–Liquid Interfaces. Adv. Mater. Interfaces 2020, 7, 200391.
- 66 Lee, K. Y.; Bae, Y.; Kim, M.; Cheong, G. W.; Kim, J.; Lee, S. S.; Han, S. W. Crown Ether Derivatives-Mediated Self-Assembly of Nanoparticles at the Liquid/Liquid Interface. Thin Solid Films 2006, 515, 2049−2054.
- 67 Xu, Y.; Konrad, M. P.; Lee, W. W. Y.; Ye, Z.; Bell, S. E. J. A Method for Promoting Assembly of Metallic and Nonmetallic Nanoparticles into Interfacial Monolayer Films. Nano Let. 2016, 16, 5255−5260.
- 68 Bian, T.; Gardin, A.; Gemen, J.; Houben, L.; Perego, C.; Lee, B.; Elad, N.; Pavan, G. M.; Klajn, R. Electrostatic Co-Assembly of Nanoparticles with oppositely Charged Small Molecules into Static and Dynamic Superstructures. Nat. Chem. 2021, 13, 940−949.
- 69 Wang, M.; Zhang, Z.; He, J. A SERS study on the Assembly Behavior of Gold Nanoparticles at the Oil/Water Interface. Langmuir 2015, 31, 12911−12919.
- 70 Fang, P. P.; Chen, S.; Deng, H.; Scanlon, M. D.; Gumy, F.; Lee, H. J.; Momotenko, D.; Amstutz, V.; Cortés-Salazar, F.; Pereira, C. M.; Yang, Z.; Girault, H. H. Conductive Gold Nanoparticle Mirrors at Liquid/Liquid Interfaces. ACS Nano 2013, 7, 9241−9248.
- 71 Bera, M. K.; Chan, H.; Moyano, D. F.; Yu, H.; Tatur, S.; Amoanu, D.; Bu, W.; Rotello, V. M.; Meron, M.; Kral, P.; Lin, B.; Schlossman, M. L. Interfacial Localization and Voltage-Tunable Arrays of Charged Nanoparticles. Nano Let. 2014, 14, 6816−6822.
- 72 Velleman, L.; Sikdar, D.; Turek, V. A.; Kucernak, A. R.; Roser, S. J.; Kornyshev, A. A.; Edel, J. B. Tuneable 2D Self-Assembly of Plasmonic Nanoparticles at Liquid|Liquid Interfaces. Nanoscale 2016, 8, 19229−19241.
- 73 Montelongo, Y.; Sikdar, D.; Ma, Y.; McIntosh, A. J.; Velleman, L.; Kucernak, A. R.; Edel, J. B.; Kornyshev, A. A. Electrotunable Nanoplasmonic Liquid Mirror. Nat. Mater. 2017, 16, 1127−1135.
- 74 Kim, K.; Han, H. S.; Choi, I.; Lee, C.; Hong, S.; Suh, S. H.; Lee, L. P.; Kang, T. Interfacial Liquid-State Surface-Enhanced Raman Spectroscopy. Nat. Commun. 2013, 4, 1−9.
- 75
Koh, C. S. L.; Lee, H. K.; Phan-Quang, G. C.; Han, X.; Lee, M. R.; Yang, Z.; Ling, X. Y. SERS-and Electrochemically Active 3D Plasmonic Liquid Marbles for Molecular-Level Spectroelectrochemical Investigation of Microliter Reactions. Angew. Chem. Int. Ed. 2017, 129, 8939–8943.
10.1002/ange.201704433 Google Scholar
- 76 Du, S.; Su, M.; Wang, C.; Ding, Z.; Jiang, Y.; Liu, H. Pinpointing Alkane Chain Length, Saturation, and Double Bond Regio-and Stereoisomers by Liquid Interfacial Plasmonic Enhanced Raman Spectroscopy. Anal. Chem. 2022, 94, 2891−2900.
- 77 Tilman, D.; Cassman, K. G.; Matson, P. A.; Naylor, R.; Polasky, S. Agricultural Sustainability and Intensive Production Practices. Nature 2002, 418, 671−677.
- 78 Wang, K.; Sun, D. W.; Pu, H.; Wei, Q. Two-Dimensional Au@Ag Nanodot Array for Sensing Dual-Fungicides in Fruit Juices with Surface-Enhanced Raman Spectroscopy Technique. Food Chem. 2020, 310, 125923.
- 79 Yu, F.; Su, M.; Tian, L.; Wang, H.; Liu, H. Organic Solvent as Internal Standards for Quantitative and High-Throughput Liquid Interfacial SERS Analysis in Complex Media. Anal. Chem. 2018, 90, 5232−5238.
- 80 Du, S.; Su, M.; Jiang, Y.; Yu, F.; Xu, Y.; Lou, X.; Yu, T.; Liu, H. Direct Discrimination of Edible Oil Type, Oxidation, and Adulteration by Liquid Interfacial Surface-Enhanced Raman Spectroscopy. ACS Sens. 2019, 4, 1798−1805.
- 81 Zhang, Q. M.; Li, D.; Cao, X. K.; Gu, H. X.; Deng, W. Self-assembled Microgels Arrays for Electrostatic Concentration and Surface-Enhanced Raman Spectroscopy Detection of Charged Pesticides in Seawater. Anal. Chem. 2019, 91, 11192−11199.
- 82 Kelly, J.; Patrick, R.; Patrick, S.; Bell, S. E. J. Surface-Enhanced Raman Spectroscopy for the Detection of a Metabolic Product in the Headspace Above Live Bacterial Cultures. Angew. Chem. Int. Ed. 2018, 57, 15686−15690.
- 83 Tian, T.; Yi, J.; Liu, Y.; Li, B.; Liu, Y.; Qiao, L.; Zhang, K.; Liu, B. Self-assembled Plasmonic Nanoarrays for Enhanced Bacterial Identification and Discrimination. Biosens. Bioelectron. 2022, 197, 113778.
- 84 Luo, W.; Wu, C; Huang, S.; Yuan, R.; Yang, X. Liquid Phase Interfacial Surface-Enhanced Raman Scattering Platform for Ratiometric Detection of MicroRNA 155. Anal. Chem. 2020, 92, 15573−15578.
- 85 Wu, C.; Huang, S.; Wang, Y.; Chai, Y.; Yuan, R.; Yang, X. DNA Structure-Stabilized Liquid–Liquid Self-Assembled Ordered Au Nanoparticle Interface for Sensitive Detection of MiRNA 155. Anal. Chem. 2021, 93, 11019−11024.
- 86 Tian, T.; Yi, J.; Liu, Y.; Li, B.; Liu, Y.; Qiao, L.; Zhang, K.; Liu, B. Self-Assembled Plasmonic Nanoarrays for Enhanced Bacterial Identification and Discrimination. Biosens. Bioelectron. 2022, 197, 113778.
- 87 Fan, G.; Gao, X.; Xu, S.; Li, X.; Zhang, Q.; Dai, C.; Xue, Q.; Wang, H. Engineering an Au Nanostar-Based Liquid Phase Interfacial Ratiometric SERS Platform with Programmable Entropy-Driven DNA Circuits to Detect Protein Biomarkers in Clinical Samples. Chem. Commun. 2022, 58, 407−410.
- 88 Zhang, M.; Li, X.; Pan, J.; Zhang, Y.; Zhang, L.; Wang, C.; Yan, X.; Liu, X.; Lu, G. Ultrasensitive Detection of SARS-CoV-2 Spike Protein in Untreated Saliva Using SERS-Based Biosensor. Biosens. Bioelectron. 2021, 190, 113421.
- 89 Lin, X.; Fang, G.; Liu, Y.; He, Y.; Wang, L.; Dong, B. Marangoni Effect-Driven Transfer and Compression at Three-Phase Interfaces for Highly Reproducible Nanoparticle Monolayers. J. Phys. Chem. Lett. 2020, 11, 3573−3581.
- 90 Kalia, L. V.; Lang, A. E. Parkinson's Disease. Lancet 2015, 386, 896–912.
- 91 Patriarchi, T.; Cho, J. R.; Merten, K.; Howe, M. W.; Marley, A.; Xiong, W. H.; Folk, R. W.; Broussard, G. J.; Liang, R.; Jang, M. J.; Zhong, H.; Dombeck, D.; Zastrow, M. v.; Nimmerjahn, A.; Gradinaru, V.; Williams, J. T.; Tian, L. Ultrafast Neuronal Imaging of Dopamine Dynamics with Designed Genetically Encoded Sensors. Science 2018, 360, eaat4422.
- 92 Mei, Y.; Zhang, Q. W.; Gu, Q.; Liu, Z.; He, X.; Tian, Y. Pillar [5] Arene-Based Fluorescent Sensor Array for Biosensing of Intracellular Multi-Neurotransmitters through Host–Guest Recognitions. J. Am. Chem. Soc. 2022, 144, 2351−2359.
- 93 Liu, Z.; Zhu, Y.; Zhang, L.; Jiang, W.; Liu, Y.; Tang, Q.; Cai, X.; Li, J.; Wang, L.; Tao, C.; Yin, X.; Li, X.; Hou, S.; Jiang, D.; Liu, K.; Zhou, X.; Zhang, H.; Liu, M.; Fan, C.; Tian, Y. Structural and Functional Imaging of Brains. Sci. China Chem. 2022, DOI: https://doi.org/10.1007/s11426-022-1408-5.
- 94 Zhou, B.; Li, X.; Tang, X.; Li, P.; Yang, L.; Liu, J. Highly Selective and Repeatable Surface-Enhanced Resonance Raman Scattering Detection for Epinephrine in Serum Based on Interface Self-Assembled 2D Nanoparticles Arrays. ACS Appl. Mater. Interfaces 2017, 9, 7772−7779.
- 95 Shi, L.; Liu, M.; Zhang, L.; Tian, Y. A Liquid Interfacial SERS Platform on a Nanoparticle Array Stabilized by Rigid Probes for the Quantification of Norepinephrine in Rat Brain Microdialysates. Angew. Chem. Int. Ed. 2022, e202117125.




