Electrochemical Selective Oxidative Synthesis of Diversified Sulfur Heterocycles from β-Ketothioamides
Li-Rong Wen
State Key Laboratory Base of Eco-Chemical Engineering, College of Chemistry and Molecular Engineering, Qingdao University of Science & Technology, Qingdao, Shandong, 266042 China
Search for more papers by this authorNing-Ning Wang
State Key Laboratory Base of Eco-Chemical Engineering, College of Chemistry and Molecular Engineering, Qingdao University of Science & Technology, Qingdao, Shandong, 266042 China
Search for more papers by this authorWu-Bo Du
State Key Laboratory Base of Eco-Chemical Engineering, College of Chemistry and Molecular Engineering, Qingdao University of Science & Technology, Qingdao, Shandong, 266042 China
Search for more papers by this authorMing-Zhe Zhu
State Key Laboratory Base of Eco-Chemical Engineering, College of Chemistry and Molecular Engineering, Qingdao University of Science & Technology, Qingdao, Shandong, 266042 China
Search for more papers by this authorChao Pan
State Key Laboratory Base of Eco-Chemical Engineering, College of Chemistry and Molecular Engineering, Qingdao University of Science & Technology, Qingdao, Shandong, 266042 China
Search for more papers by this authorCorresponding Author
Lin-Bao Zhang
State Key Laboratory Base of Eco-Chemical Engineering, College of Chemistry and Molecular Engineering, Qingdao University of Science & Technology, Qingdao, Shandong, 266042 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Ming Li
State Key Laboratory Base of Eco-Chemical Engineering, College of Chemistry and Molecular Engineering, Qingdao University of Science & Technology, Qingdao, Shandong, 266042 China
E-mail: [email protected]; [email protected]Search for more papers by this authorLi-Rong Wen
State Key Laboratory Base of Eco-Chemical Engineering, College of Chemistry and Molecular Engineering, Qingdao University of Science & Technology, Qingdao, Shandong, 266042 China
Search for more papers by this authorNing-Ning Wang
State Key Laboratory Base of Eco-Chemical Engineering, College of Chemistry and Molecular Engineering, Qingdao University of Science & Technology, Qingdao, Shandong, 266042 China
Search for more papers by this authorWu-Bo Du
State Key Laboratory Base of Eco-Chemical Engineering, College of Chemistry and Molecular Engineering, Qingdao University of Science & Technology, Qingdao, Shandong, 266042 China
Search for more papers by this authorMing-Zhe Zhu
State Key Laboratory Base of Eco-Chemical Engineering, College of Chemistry and Molecular Engineering, Qingdao University of Science & Technology, Qingdao, Shandong, 266042 China
Search for more papers by this authorChao Pan
State Key Laboratory Base of Eco-Chemical Engineering, College of Chemistry and Molecular Engineering, Qingdao University of Science & Technology, Qingdao, Shandong, 266042 China
Search for more papers by this authorCorresponding Author
Lin-Bao Zhang
State Key Laboratory Base of Eco-Chemical Engineering, College of Chemistry and Molecular Engineering, Qingdao University of Science & Technology, Qingdao, Shandong, 266042 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Ming Li
State Key Laboratory Base of Eco-Chemical Engineering, College of Chemistry and Molecular Engineering, Qingdao University of Science & Technology, Qingdao, Shandong, 266042 China
E-mail: [email protected]; [email protected]Search for more papers by this authorMain observation and conclusion
A general and practical protocol for the construction of diversified sulfur heterocycles has been described through organic electrosynthesis means. In undivided cell, dihydrothiophenes, thiazolines and 1,4-dithiines could be easily generated from various available β-ketothioamides under metal-free and external oxidant-free conditions. The transformation underwent smoothly under mild conditions and could be easily scaled-up. Moreover, different sulfur heterocycles were generated through varying solvent and 1,4-diazabicyclo[2.2.2]octane (DABCO) could enable the hydrogen atom transfer (HAT) process of this transformation.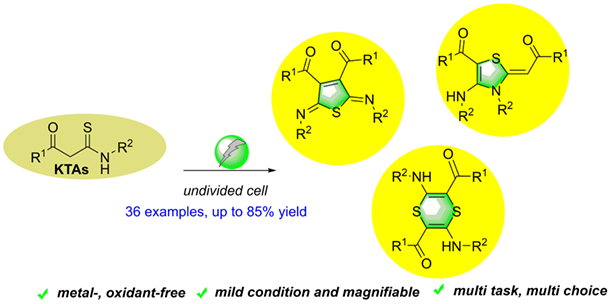
Supporting Information
| Filename | Description |
|---|---|
| cjoc202100132-sup-0001-Supinfo.pdfPDF document, 12.1 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1(a) Kumar, A.; Rajput, C. S.; Bhati, S. K. Synthesis of 3-[4′-(p-Chlorophenyl)-Thiazol-2′-yl]-2-[(Substituted Azetidinone/Thiazolidinone)- Aminomethyl]-6-bromoquinazolin-4-ones as AntiInflammatory Agent. Bioorg. Med. Chem. 2007, 15, 3089–3096; (b) Omar, K.; Geronikaki, A.; Zoumpoulakis, P.; Camoutsis, C.; Sokovic, M.; Ciric , A.; Glamoclija. Novel 4-Thiazolidinone Derivatives as Potential Antifungal and Antibacterial Drugs. Bioorg. Med. Chem. 2010, 18, 426–432; (c) Prakash, O.; Hussain, K.; Aneja, D. K.; Sharma, C.; Aneja, K. R. A Facile Iodine(III)-Mediated Synthesis of 3-(3-Aryl-1-phenyl-1H-pyrazol-4-yl)- [1,2,4]triazolo [4,3-a]pyridines via Oxidation of 2-((3-Aryl-1-phenyl- 1H-pyrazol-4-yl)methylene)-1-(pyridin -2-yl)hydrazines and Their Antimicrobial Evaluations. Org. Med. Chem. Lett. 2011, 1, 1–9; (d) Kucukguzel, S. G.; Oruc, E. E.; Rollas, S.; Sahin, F.; Ozbek, A. Synthesis, Characterisation and Biological Activity of Novel 4-Thiazolidinones, 1,3,4-Oxadiazoles and Some Related Compounds. Eur. J. Med. Chem. 2002, 37, 197–206; (e) Chen, H.; Guo, Z. H.; Yin, Q. M.; Duan, X. X.; Gu, Y. J.; Li, X. L. Design, Synthesis and HIV-RT Inhibitory Activity of Novel Thiazolidin-4-one Derivatives. Front. Chem. Sci. Eng. 2011, 5, 231–237; (f) Rawal, R. K.; Tripathi, R.; Katti, S. B.; Pannecouque, C.; Clercq, E. D. Design, Synthesis, and Evaluation of 2-Aryl-3-heteroaryl- 1,3-thiazolidin-4-ones as Anti-HIV Agents. Bioorg. Med. Chem. 2007, 15, 1725–1731; (g) Feng, M.; Tang, B.; Liang, S. H.; Jiang, X. Sulfur containing scaffolds in drugs: synthesis and application in medicinal chemistry. Curr. Top. Med. Chem. 2016, 16, 1200–1216.
- 2(a) Kremer, L.; Douglas, J. D.; Baulard, A. R.; Morehouse, C.; Guy, M. R.; Alland, D.; Dover, L. G.; Lakey, J. H.; Jacobs, W. R.; Brennan, P. J.; Minnikin, D. E.; Besra, G. S. Thiolactomycin and Related Analogues as Novel Anti-mycobacterial Agents Targeting KasA and KasB Condensing Enzymes in Mycobacterium tuberculosis. J. Biol. Chem. 2000, 275, 16857–16864; (b) Schiebel, J.; Kapilashrami, K.; Fekete, A.; Bommineni, G. R.; Schaefer, C. M.; Mueller, M. J.; Tonge, P. J.; Kisker, C. Structural Basis for the Recognition of Mycolic Acid Precursors by KasA, a Condensing Enzyme and Drug Target from Mycobacterium Tuberculosis. J. Biol. Chem. 2013, 288, 34190–34204.
- 3(a) Kao, J.; Lilly, A. C. Jr. Theoretical Studies of Electronic Properties of Polyacene, Poly(1,4-dihydrobenzo-1,4-dihydrobenzene), poly(p- phenylene), poly(p-1,4-dihydrobenzene), and Their Hetero (N, O, and S) Substituted Derivatives. J. Am. Chem. Soc. 1987, 109, 4149–4157; (b) Bryce, M. R.; Chesney, A.; Lay, A. K.; Batsanov, A. S.; Howard, J. A. K. New π-Electron Donor Systems Based on Acenaphtho[1,2-b][1,4]dithiine. J. Chem. Soc., Perkin Trans. 1 1996, 2451–2459; (c) Peintinger, M. F.; Beck, J.; Bredow, T. Charged Stacks of Dithiin, Diselenin, Thianthrene and Selenanthrene Radical Cations: Long Range Multicenter Bonds. Phys. Chem. Chem. Phys. 2013, 15, 18702–18709.
- 4(a) Dunbar, K. L.; Scharf, D. H.; Litomska, A.; Hertweck, C. Enzymatic Carbon–Sulfur Bond Formation in Natural Product Biosynthesis. Chem. Rev. 2017, 117, 5521–5577; (b) Guo, X. X.; Gu, D. W.; Wu, Z. X.; Zhang, W. B. Copper-Catalyzed C–H Functionalization Reactions: Efficient Synthesis of Heterocycles. Chem. Rev. 2015, 115, 1622–1651; (c) Ogiwara, Y.; Takano, K.; Horikawa, S.; Sakai, N. Indium-Catalyzed Direct Conversion of Lactones into Thiolactones Using a Disilathiane as a Sulfur Source. Molecules 2018, 23, 1339–1349; (d) Edwards, G. A.; Culp, P. A.; Chalker, J. M. Allyl Sulphides in Olefin Metathesis: Catalyst Considerations and Traceless Promotion of Ring-closing Metathesis. Chem. Commun. 2015, 51, 515–518; (e) Peng, Y. Y.; He, Q.; Zhang, X. F.; Yang, C. H. Pd-Catalyzed Intramolecular C–H Activation and C–S Formation to Synthesize Pyrazolo[5,1-b]benzothiazoles without an Additional Oxidant. Org. Chem. Front. 2019, 6, 3234–3237; (f) Heravi, M. M.; Kivanloo, A.; Rahimizadeh, M.; Bakavoli, M.; Ghassemzadeh, M.; Neumuller, B. Regioselective Synthesis of 3-Benzylthiazolo[3,2-a]pyrimidones and 3-Benzyl-thiazolo[3,2-c]pyrimidones through Palladium-Catalyzed Heteroannulation of Acetylenic Compounds. Phosphorus, Sulfur, Silicon 2005, 180, 2407–2417; (g) Arisawa, M.; Ichikawa, T.; Tanii, S.; Yamaguchi, M. Synthesis of Symmetrical and Unsymmetrical 1,4-Dithiins by Rhodium-Catalyzed Sulfur Addition Reaction to Alkynes. Synthesis 2016, 48, 3107–3119; (h) Tan, W.; Jansch, N.; Öhlmann, T.; Meyer-Almes, F.-J.; Jiang, X. Thiocarbonyl surrogate via combination of potassium sulfide and chloroform for dithiocarbamate construction. Org. Lett. 2019, 21, 7484–7488; (i) Tan, W.; Wang, C.; Jiang, X. Visible-light-mediated C(sp3)–H thiocarbonylation for thiolactam preparation with potassium sulfide. Chin. J. Chem. 2019, 37, 1234–1238; (j) Tan, W.; Wang, C.; Jiang, X. Green carbon disulfide surrogate via a combination of potassium sulfide and chloroform for benzothiazine-thione and benzothiazolethione construction. Org. Chem. Front. 2018, 5, 2390–2394.
- 5 Guo, W. S.; Wen, L. R.; Li, M. β-Ketothioamides: Efficient Reagents in the Synthesis of Heterocycles. Org. Biomol. Chem. 2015, 13, 1942–1953.
- 6(a) Ansari, M. A.; Yadav, D.; Soni, S.; Srivastava, A.; Singh, M. S. Visible-Light-Mediated Synthesis of 1,2,4-Dithiazolidines from β-Ketothioamides through a Hydrogen-Atom-Transfer Photocatalytic Approach of Eosin Y. J. Org. Chem. 2019, 84, 5404–5412; (b) Ramulu, B. J.; Nagaraju, A.; Chowdhury, S.; Koley, S.; Singh, M. S. Metal–Free Reagent Dependent S—S and C—C Homocoupling of α-Enolic Dithioesters at Room Temperature: Direct Access to Fully Substituted Symmetrical Thiophenes via Chemoselective Paal–Knorr Approach. Adv. Synth. Catal. 2015, 357, 530–538; (c) Ansari, M. A.; Yadav, D.; Soni, S.; Singh, M. S. Phosphonium Ylide Catalysis: A Divergent Diastereoselective Approach to Synthesize Cyclic Ketene Acetals [Thia(zolidines/zinanes)] from β-Ketothioamides and Dihaloalkanes. Org. Biomol. Chem. 2019, 17, 9151–9162; (d) Yadav, D.; Shukla, G.; Ansari, M. A.; Srivastava, A.; Singh, M. S. Chemoselective One-pot Access to Benzo[e]indole-4,5-diones and Naphtho[2,1-b]thiophene- 4,5-diones via Copper-Catalyzed Oxidative [3 + 2] Annulation of α-Oxoketene N,S-Acetals/β-Ketothioamides with α-/β-Naphthols. Tetrahedron 2018, 74, 5920–5931; (e) Ansari, M. A.; Yadav, D.; Singh, M. S. Rhodium(II)-Catalyzed Annulative Coupling of β-Ketothioamides with α-Diazo Compounds: Access to Highly Functionalized Thiazolidin-4-ones and Thiazolines. J. Org. Chem. 2020, 85, 8320–8329; (f) Ansari, M. A.; Yadav, D.; Singh, M. S. Visible-Light-Driven Photocatalyst- and Additive-Free Cross-Coupling of β-Ketothioamides with α-Diazo 1,3-Diketones: Access to Highly Functionalized Thiazolines. Chem. − Eur. J. 2020, 26, 8083–8089; (g) Soni, S.; Pali, P.; Ansari, M. A.; Singh, M. S. Visible-Light Photocatalysis of Eosin Y: HAT and Complementing MS-CPET Strategy to Trifluoromethylation of β-Ketodithioesters with Langlois’ Reagent. J. Org. Chem. 2020, 85, 10098–10109; (h) Srivastava, A.; Shukla, G.; Yadav, D.; Singh, M. S. Access to Fully Substituted Thiazoles and 2,3-Dihydrothiazoles via Copper-Catalyzed [4 + 1] Heterocyclization of α-(N-Hydroxy/aryl)imino-β-oxodithioesters with α-Diazocarbonyls. J. Org. Chem. 2017, 82, 10846–10854.
- 7(a) Han, T.; Wang, Y.; Li, H. L.; Luo, X. Y.; Deng, W. P. Synthesis of Polysubstituted 3-Aminothiophenes from Thioamides and Allenes via Tandem Thio-Michael Addition/Oxidative Annulation and 1,2-Sulfur Migration. J. Org. Chem. 2018, 83, 1538–1542; (b) Luo, X. Y.; Ge, L. S.; An, X. L.; Jin, J. H.; Wang, Y.; Sun, P. P.; Deng, W. P. Regioselective Metal-Free One-Pot Synthesis of Functionalized 2-Aminothiophene Derivatives. J. Org. Chem. 2015, 80, 4611–4617; (c) Wen, M.; Sun, P. P.; Luo, X. Y.; Deng, W. P. Cu(II)-Catalyzed One-Pot Synthesis of Fully Substituted Dihydrothiophenes and Thiophenes from Thioamides and Enynones. Tetrahedron 2018, 74, 4168–4173; (d) Zeng, D. M.; Wang, M.; Deng, W. P.; Jiang, X. F. The Same Oxygenation-state Introduction of Hypervalent Sulfur under Transition-Metal-Free Conditions. Org. Chem. Front. 2020, 7, 3956–3966.
- 8 Nandi, G. C.; Soumini, K. Catalyst-Controlled Straightforward Synthesis of Highly Substituted Pyrroles/Furans via Propargylation/Cycloisomerization of α-Oxoketene-N,S-acetals. J. Org. Chem. 2016, 81, 11909–11915.
- 9(a) Guo, W. S.; Gong, H.; Zhang, Y. A.; Wen, L. R.; Li, M. Fast Construction of 1,3-Benzothiazepines by Direct Intramolecular Dehydrogenative C–S Bond Formation of Thioamides under Metal-Free Conditions. Org. Lett. 2018, 20, 6394–6397; (b) Li, M.; Cao, H.; Wang, Y.; Lv, X. L.; Wen, L. R. One-Pot Multicomponent Cascade Reaction of N,S-Ketene Acetal: Solvent-Free Synthesis of Imidazo[1,2-a]thiochromeno[3,2-e]pyridines. Org. Lett. 2012, 14, 3470–3473; (c) Li, C. X.; Liu, R. J.; Yin, K.; Wen, L. R.; Li, M. Synthesis of Disulfides Tethered Pyrroles from β-Ketothioamides via a Bicyclization/Ring-opening/Oxidative Coupling Reaction. Org. Biomol. Chem. 2017, 15, 5820–5823; (d) Man, N. N.; Wang, J. Q.; Zhang, L. M.; Wen, L. R.; Li, M. Chemo-, Regio-, and Stereoselective Construction of Core Skeleton of Furo[2,3- b]pyrrole via Multicomponent Bicyclization Reaction. J. Org. Chem. 2017, 82, 5566–5573; (e) Wen, L. R.; Man, N. N.; Yuan, W. K.; Li, M. Direct Construction of 2-Aryliminochromenes from Arynes, N, S-Keteneacetals, and DMF. J. Org. Chem. 2016, 81, 5942–5948; (f) Li, M.; Kong, X. J.; Wen, L. R. Yb(OTf)3-Mediated Access to Furans from β-Ketothioamides via Eschenmoser Sulfide Contraction Reaction. J. Org. Chem. 2015, 80, 11999–12005.
- 10 Ansari, M. A.; Yadav, D.; Soni, S.; Srivastava, A.; Singh, M. S. Visible- Light-Mediated Synthesis of 1,2,4-Dithiazolidines from β-Ketothioamides through a Hydrogen-Atom-Transfer Photocatalytic Approach of Eosin Y. J. Org. Chem. 2019, 84, 5404–5412.
- 11(a) Xiong, P.; Xu, H. C. Chemistry with Electrochemically Generated N-Centered Radicals. Acc. Chem. Res. 2019, 52, 3339–3350; (b) Hou, Z. W.; Mao, Z. Y.; Xu, H. C. Recent Progress on the Synthesis of (Aza)indoles through Oxidative Alkyne Annulation Reactions. Synlett 2017, 28, 1867–1872; (c) Meyer, T. H.; Choi, I.; Tian, C.; Ackermann, L. Powering the Future: How Can Electrochemistry Make a Difference in Organic Synthesis. Chem 2020, 6, 2484–2496; (d) Wang, H.; Gao, X.; Lv, Z.; Abdelilah, T.; Lei, A. W. Recent Advances in Oxidative R1-H/R2-H Cross-Coupling with Hydrogen Evolution via Photo-/Electrochemistry. Chem. Rev. 2019, 119, 6769–6787; (e) Wang, P.; Gao, X. L.; Huang, P. F.; Lei, A. W. Recent Advances in Electrochemical Oxidative Cross-Coupling of Alkenes with H2 Evolution. ChemCatChem 2020, 12, 27–40; (f) Ma, C.; Fang, P.; Mei, T. S. Recent Advances in C–H Functionalization Using Electrochemical Transition Metal Catalysis. ACS Catal. 2018, 8, 7179–7189; (g) Shi, S. H.; Liang, Y.; Jiao, N. Electrochemical Oxidation Induced Selective C–C Bond Cleavage. Chem. Rev. 2021, 121, 485–505; (h) Sauer, G. S.; Lin, S. An Electrocatalytic Approach to the Radical Difunctionalization of Alkenes. ACS Catal. 2018, 8, 5175–5187; (i) Jiang, Y. Y.; Xu, K.; Zeng, C. C. Use of Electrochemistry in the Synthesis of Heterocyclic Structures. Chem. Rev. 2018, 118, 4485–4540; (j) Juzeliu̅nas, E.; Fray, D. J. Silicon Electrochemistry in Molten Salts. Chem. Rev. 2020, 120, 1690–1709; (k) Yan, M.; Kawamata, Y.; Baran, P. S. Synthetic Organic Electrochemical Methods Since 2000: On the Verge of a Renaissance. Chem. Rev. 2017, 117, 13230–13319; (l) Ye, Z.; Zhang, F. Recent advances in constructing Nitrogen-containing heterocycles via electrochemical dehydrogenation. Chin. J. Chem. 2019, 37, 513–528; (m) Huang, C.; Xu, H.-C. Synthesis of 1,3-benzothiazines by intramolecular dehydrogenative C–S cross-coupling in a flow electrolysis cell. Sci. China Chem. 2019, 62, 1501–1503.
- 12 Qian, X. Y.; Li, S. Q.; Song, J.; Xu, H. C. TEMPO-Catalyzed Electrochemical C–H Thiolation: Synthesis of Benzothiazoles and Thiazolopyridines from Thioamides. ACS Catal. 2017, 7, 2730–2734.
- 13(a) Huang, C.; Qian, X. Y.; Xu, H. C. Continuous-Flow Electrosynthesis of Benzofused S-Heterocycles by Dehydrogenative C−S Cross-Coupling. Angew. Chem. Int. Ed. 2019, 58, 6650–6653; (b) Folgueiras- Amador, A. A.; Qian, X. Y.; Xu, H. C.; Wirth, T. Catalyst-and Supporting Electrolyte-Free Electrosynthesis of Benzothiazoles and Thiazolopyridines in Continuousf Flow. Chem. − Eur. J. 2018, 24, 487–491.
- 14 Wang, P.; Tang, S.; Lei, A. W. Electrochemical Intramolecular Dehydrogenative C–S Bond Formation for the Synthesis of Benzothiazoles. Green Chem. 2017, 19, 2092–2095.
- 15(a) Wang, H. B.; Wang, L.; Shang, J. S.; Li, X.; Wang, H. Y.; Gui, J.; Lei, A. W. Fe-Catalysed Oxidative C–H Functionalization/C–S Bond Formation. Chem. Commun. 2012, 48, 76–78; (b) Rey, V.; Soria-Castro, S. M.; Argüello, J. E.; Peñeńory, A. B. Photochemical Cyclization of Thioformanilides by Chloranil: An Approach to 2-Substituted Benzothiazoles. Tetrahedron Lett. 2009, 50, 4720–4723; (c) Cheng, Y. N.; Yang, J.; Qu, Y.; Li, P. X. Aerobic Visible-Light Photoredox Radical C–H Functionalization: Catalytic Synthesis of 2-Substituted Benzothiazoles. Org. Lett. 2012, 14, 98–101; (d) Gan, Z.; Li, G.; Yang, X.; Yan, Q.; Xu, G.; Li, G.; Jiang, Y. Y.; Yang, D. Visible-Light-Induced Regioselective Cross-Dehydrogenative Coupling of 2-Isothiocyanatonaphthalenes with Amines Using Molecular Oxygen. Sci. China Chem. 2020, 63, 1652–1658.
- 16 Wang, P.; Tang, S.; Huang, P. F.; Lei, A. W. Electrocatalytic Oxidant- Free Dehydrogenative C−H/S−H Cross-Coupling. Angew. Chem. Int. Ed. 2017, 56, 3009–3013.
- 17(a) Zhang, Y. A.; Ding, Z.; Liu, P.; Guo, W. S.; Wen, L. R.; Li, M. Access to SCN-Containing Thiazolines via Electrochemical Regioselective Thiocyanothiocyclization of N-Allylthioamides. Org. Chem. Front. 2020, 7, 1321–1326; (b) Zhang, L. B.; Geng, R. S.; Wang, Z. C.; Ren, G. Y.; Wen, L. R.; Li, M. Electrochemical Intramolecular C–H/N–H Functionalization for the Synthesis of Isoxazolidine-Fused Isoquinolin- 1(2H)-ones. Green Chem. 2020, 22, 16–21; (c) Wen, L. R.; Sun, Y. X.; Zhang, J. W.; Guo, W. S.; Li, M. Catalyst- and Solvent-Free Bisphosphinylation of Isothiocyanates: a Practical Method for the Synthesis of Bisphosphinoylaminomethanes. Green Chem. 2018, 20, 125–129; (d) Wen, L. R.; Li, Z. R.; Li, M.; Cao, H. Solvent-Free and Efficient Synthesis of Imidazo[1,2-a]pyridine Derivatives via a One-Pot Three- Component Reaction. Green Chem. 2012, 14, 707–716.
- 18 Ye, Z.; Wu, Y.; Chen, N.; Zhang, H.; Zhu, K.; Ding, M.; Liu, M.; Li, Y.; Zhang, F. Z. Enantiospecific Electrochemical Rearrangement for the Synthesis of Hindered Triazolopyridinone Derivatives. Nat. Commun. 2020, 11, 3628–3636.
- 19(a) Jeffrey, J. L.; Petronijević, F. R.; MacMillan, D. W. C. Selective Radical–Radical Cross-Couplings: Design of a Formal β-Mannich Reaction. J. Am. Chem. Soc. 2015, 137, 8404–8407; (b) Twilton, J.; Christensen, M.; DiRocco, D. A.; Ruck, R. T.; Davies, I. W.; MacMillan, D. W. C. Selective Hydrogen Atom Abstraction through Induced Bond Polarization: Direct α-Arylation of Alcohols through Photoredox, HAT, and Nickel Catalysis. Angew. Chem. Int. Ed. 2018, 57, 5369–5373; (c) Shaw, M. H.; Shurtleff, V. W.; Terrett, J. A.; Cuthbertson, J. D.; MacMillan, D. W. C. A Highly Efficient Directional Molecular White-Light Emitter Driven by a Continuous-Wave laser Diode. Science 2016, 352, 1301–1304.




