Clerodenoids A—F: C-ring Aromatized and/or Rearranged Abietane Diterpenoids from Clerodendrum chinense var. simplex
Jingjing Qi
School of Chinese Materia Medica, Nanjing University of Chinese Medicine, 138 Xianlin Road, Nanjing, Jiangsu, 210023 China
Search for more papers by this authorYan Zhang
State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, 555 Zu Chong Zhi Road, Shanghai, 201203 China
Search for more papers by this authorQunfang Liu
State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, 555 Zu Chong Zhi Road, Shanghai, 201203 China
Search for more papers by this authorCorresponding Author
Hongchun Liu
State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, 555 Zu Chong Zhi Road, Shanghai, 201203 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Yaoyue Fan
State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, 555 Zu Chong Zhi Road, Shanghai, 201203 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Jianmin Yue
School of Chinese Materia Medica, Nanjing University of Chinese Medicine, 138 Xianlin Road, Nanjing, Jiangsu, 210023 China
State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, 555 Zu Chong Zhi Road, Shanghai, 201203 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorJingjing Qi
School of Chinese Materia Medica, Nanjing University of Chinese Medicine, 138 Xianlin Road, Nanjing, Jiangsu, 210023 China
Search for more papers by this authorYan Zhang
State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, 555 Zu Chong Zhi Road, Shanghai, 201203 China
Search for more papers by this authorQunfang Liu
State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, 555 Zu Chong Zhi Road, Shanghai, 201203 China
Search for more papers by this authorCorresponding Author
Hongchun Liu
State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, 555 Zu Chong Zhi Road, Shanghai, 201203 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Yaoyue Fan
State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, 555 Zu Chong Zhi Road, Shanghai, 201203 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Jianmin Yue
School of Chinese Materia Medica, Nanjing University of Chinese Medicine, 138 Xianlin Road, Nanjing, Jiangsu, 210023 China
State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, 555 Zu Chong Zhi Road, Shanghai, 201203 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorMain observation and conclusion
As part of our continuing efforts toward the discovery of the biologically active diterpenoids from medicinal plants, six new C-ring aromatized abietane diterpenoids, clerodenoids A—F (1—6), were isolated from Clerodendrum chinense. These C-ring aromatized compounds mainly belonged to the rearranged abietane skeletons of 17(15→16)-abeo-abietane (2—4) and 17(15→16), 18(4→3)-diabeo-abietane (5 and 6). Their structures were elucidated by extensive spectroscopic data, X-ray crystallography, and ECD calculation. Compounds 5 and 6 displayed strong antiproliferative activities against A549 and HL-60 cell lines.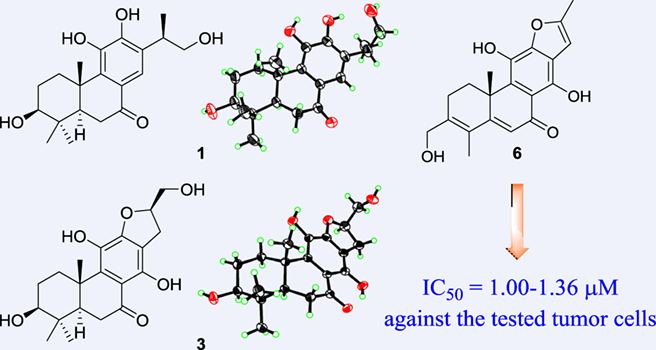
Supporting Information
| Filename | Description |
|---|---|
| cjoc202100117-sup-0001-Supinfo.pdfPDF document, 9.1 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1Flora of China Editorial Committee. In Flora Reipublicae Popularis Sinicae, Vol. 65, Science Press, Beijing , 1982, p. 174.
- 2 Tian, X. D.; Min, Z. D.; Xie, N.; Lei, Y.; Tian, Z. Y.; Zheng, Q. T.; Xu, R. N.; Tanaka, T.; Iinuma, M.; Mizuno, M. Abietane diterpenes from Clerodendron cyrtophyllum. Chem. Pharm. Bull. 1993, 41, 1415–1417.
- 3 Kang, D. G.; Lee, Y. S.; Kim, H. J.; Lee, Y. M.; Lee, H. S. Angiotensin converting enzyme inhibitory phenylpropanoid glycosides from Clerodendron trichotomum. J. Ethnopharmacol. 2003, 89, 151–154.
- 4 Xu, M. F.; Shen, L. Q.; Wang, K. W.; Du, Q. Z.; Wang, N. A new rearranged abietane diterpenoid from Clerodendrum kaichianum Hsu. J. Asian Nat. Prod. Res. 2011, 13, 260–264.
- 5 Lee, J. Y.; Lee, J. G.; Sim, S. S.; Whang, W. K.; Kim, C. J. Anti-asthmatic effects of phenylpropanoid glycosides from Clerodendron trichotomum leaves and Rumex gmelini herbes in conscious guinea-pigs challenged with aerosolized ovalbumin. Phytomedicine 2011, 18, 134–142.
- 6 Prasad, M. P.; Sushant, S.; Chikkaswamy, B. K. Phytochemical analysis, antioxidant potential, antibacterial activity and molecular characterization of Clerodendrum species. Int. J. Mol. Biol. 2012, 3, 71–76.
- 7 Li, L. Z.; Wu, L.; Wang, M. H.; Sun, J. B.; Liang, J. Y. Abietane diterpenoids from Clerodendrum trichotomum and correction of NMR data of villosin C and B. Nat. Prod. Commun. 2014, 9, 907–910.
- 8 Yue, J. R.; Feng, D. Q.; Xu, Y. K. A new triterpenoid bearing octacosanoate from the stems and roots of Clerodendrum philippinum var. simplex (Verbenaceae). Nat. Prod. Res. 2015, 29, 1228–1234.
- 9 Kar, M. K.; Swain, T. R.; Mishra, S. K. Antidiabetic activity of Clerodendrum philippinum Schauer leaves in streptozotocin induced diabetic rats. Int. J. Pharm. Pharm. Sci. 2015, 7, 386–389.
- 10 Xu, M. F.; Jia, O. Y.; Wang, S. J.; Zhu, Q. A new bioactive diterpenoid from Pestalotiopsis adusta, an endophytic fungus from Clerodendrum canescens. Nat. Prod. Res. 2016, 30, 2642–2647.
- 11 Hu, H. J.; Zhou, Y.; Han, Z. Z.; Shi, Y. H.; Zhang, S. S.; Wang, Z. T.; Yang, L. Abietane diterpenoids from the roots of Clerodendrum trichotomum and their nitric oxide inhibitory activities. J. Nat. Prod. 2018, 81, 1508–1516.
- 12Nanjing University of Chinese Medicine. Dictionary of Traditional Chinese Medicine, Shanghai Science and Technology Press, Shanghai, 2006.
- 13 Ge, Z. P.; Liu, H. C.; Wang, G. C.; Liu, Q. F.; Xu, C. H.; Ding, J.; Fan, Y. Y.; Yue, J. M. 17-nor-Cephalotane-type diterpenoids from Cephalotaxus fortunei. J. Nat. Prod. 2019, 82, 1565–1575.
- 14 Gao, X. H.; Fan, Y. Y.; Liu, Q. F.; Cho, S. H.; Pauli, G. F.; Chen, S. N.; Yue, J. M. Suadimins A—C, unprecedented dimeric quinoline alkaloids with antimycobacterial activity from Melodinus suaveolens. Org. Lett. 2019, 21, 7065–7068.
- 15 Cui, J. J.; Ji, K. L.; Liu, H. C.; Zhou, B.; Liu, Q. F.; Xu, C. H.; Ding, J.; Zhao, J. X.; Yue, J. M. Cytotoxic tigliane diterpenoids from Croton damayeshu. J. Nat. Prod. 2019, 82, 1550–1557.
- 16 Zhang, W. Y.; Zhao, J. X.; Sheng, L.; Fan, Y. Y.; Li, J. Y.; Gao, K.; Yue, J. M. Mangelonoids A and B, two pairs of macrocyclic diterpenoid enantiomers from Croton mangelong. Org. Lett. 2018, 20, 4040–4043.
- 17 Ni, G.; Zhang, H.; Fan, Y. Y.; Liu, H. C.; Ding, J.; Yue, J. M. Mannolides A-C with an intact diterpenoid skeleton providing insights on the biosynthesis of antitumor Cephalotaxus Troponoids. Org. Lett. 2016, 18, 1880–1883.
- 18 Zhou, B.; Wu, Y.; Gan, L. S.; Dalal, S.; Cassera, M. B.; Yue, J. Structurally interesting diarymethane derivatives from Securidaca inappendiculata. Chin. J. Chem. 2020, 38, 812–816.
- 19 Flack, H. D. On Enantiomorph-Polarity Estimation. Acta Crystallogr. Sect. A: Found. Crystallogr. 1983, 39, 876–881.
- 20 Flack, H. D.; Bernardinelli, G. The use of X-ray crystallography to determine absolute configuration. Chirality 2008, 20, 681–690.
- 21 Luo, G. Y.; Ye, Q.; Du, B. W.; Wang, F.; Zhang, G. L.; Luo, Y. G. Iridoid glucosides and diterpenoids from Caryopteris glutinosa. J. Nat. Prod. 2016, 79, 886–893.
- 22 Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J. T.; Bokesch, H.; Kenney, S.; Boyd, M. R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl Cancer I. 1990, 82, 1107–1112.
- 23 Tominaga, H.; Ishiyama, M.; Ohseto, F.; Sasamoto, K.; Hamamoto, T.; Suzuki, K.; Watanabe, M. A water-soluble tetrazolium salt useful for colorimetric cell viability assay. Anal. Commun. 1999, 36, 47–50.
- 24 Zhou, B.; Wu, Y.; Dalal, S.; Merino, E. F.; Liu, Q. F.; Xu, C. H.; Yuan, T.; Ding, J.; Kingston, D. G. I.; Cassera, M. B.; Yue, J. M. Nanomolar antimalarial agents against chloroquine-resistant plasmodium falciparum from medicinal plants and their structure-activity relationships. J. Nat. Prod. 2017, 80, 96–107.
- 25 Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341.
- 26 Sheldrick, G. SHELXT-Integrated space-group and crystal-structure determination. Acta Cryst. A 2015, 71, 3–8.
- 27 Sheldrick, G. Crystal structure refinement with SHELXL. Acta Cryst. C 2015, 71, 3–8.




