Asymmetric Synthesis of Chiral 1,3-Disubstituted Allylsilanes via Copper(I)-Catalyzed 1,4-Conjugate Silylation of α,β-Unsaturated Sulfones and Subsequent Julia-Kocienski Olefination
Xian-Liang Wang
CAS Key Laboratory of Synthetic Chemistry of Natural Substances, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 China
Department of Chemistry, Shanghai University, 99 Shangda Road, Shanghai, 200444 China
Search for more papers by this authorXing-Hao Yin
CAS Key Laboratory of Synthetic Chemistry of Natural Substances, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 China
Search for more papers by this authorJun-Zhao Xiao
CAS Key Laboratory of Synthetic Chemistry of Natural Substances, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 China
Search for more papers by this authorXue-Shun Jia
Department of Chemistry, Shanghai University, 99 Shangda Road, Shanghai, 200444 China
Search for more papers by this authorCorresponding Author
Liang Yin
CAS Key Laboratory of Synthetic Chemistry of Natural Substances, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 China
E-mail: [email protected]Search for more papers by this authorXian-Liang Wang
CAS Key Laboratory of Synthetic Chemistry of Natural Substances, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 China
Department of Chemistry, Shanghai University, 99 Shangda Road, Shanghai, 200444 China
Search for more papers by this authorXing-Hao Yin
CAS Key Laboratory of Synthetic Chemistry of Natural Substances, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 China
Search for more papers by this authorJun-Zhao Xiao
CAS Key Laboratory of Synthetic Chemistry of Natural Substances, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 China
Search for more papers by this authorXue-Shun Jia
Department of Chemistry, Shanghai University, 99 Shangda Road, Shanghai, 200444 China
Search for more papers by this authorCorresponding Author
Liang Yin
CAS Key Laboratory of Synthetic Chemistry of Natural Substances, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 China
E-mail: [email protected]Search for more papers by this authorMain observation and conclusion
A general synthesis of chiral 1,3-disubstituted allylsilanes is established through copper(I)-catalyzed asymmetric 1,4-conjugate silylation of α,β-unsaturated sulfones and subsequent Julia-Kocienski olefination. By modification of McQuade's NHC ligand, the catalytic asymmetric conjugate silylation with a broad substrate scope is achieved in high enantioselectivity. The following Julia-Kocienski olefination proceeds smoothly at room temperature to deliver an array of chiral allylsilanes in moderate yields. More interestingly, a one-pot asymmetric synthesis with high synthetic efficiency is successfully realized. Utility of the prepared chiral 1,3-disubsituted allylsilanes is demonstrated in the asymmetric allylation of both aldehyde and aldimine. Finally, an interesting “match and mismatch” phenomenon is observed in the asymmetric allylation of chiral aldehydes.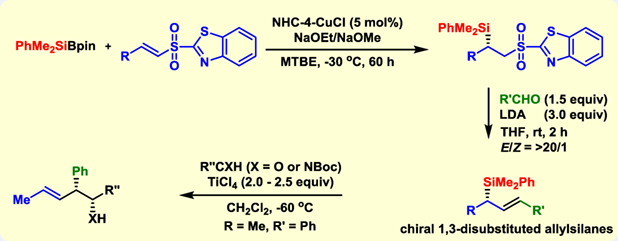
Supporting Information
| Filename | Description |
|---|---|
| cjoc202100101-sup-0001-Supinfo.pdfPDF document, 11.2 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Marshall, J. A. Chiral Allylic and Allenic Metal Reagents for Organic Synthesis. J. Org. Chem. 2007, 72, 8153–8166.
- 2 Masse, C. E.; Panek, J. S. Reactions of Chiral Allyl and Allenyl Silanes with Activated C=X π-Bonds. Chem. Rev. 1995, 95, 1293–1316.
- 3 Fleming, I.; Barbero, A.; Walter, D. Stereochemical Control in Organic Synthesis Using Silicon-Containing Compounds. Chem. Rev. 1997, 97, 2063–2192.
- 4 Chabaud, L.; James, P.; Landais, Y. Allylsilanes in Organic Synthesis - Recent Developments. Eur. J. Org. Chem. 2004, 2004, 3173–3199.
- 5 Hosomi, A.; Sakurai, H. Syntheses of γ,δ-unsaturated Alcohols from Allylsilanes and Carbonyl Compounds in the Presence of Titanium Tetrachloride. Tetrahedron Lett. 1976, 17, 1295–1298.
- 6 Hosomi, A.; Endo, M.; Sakurai, H. Allylsilanes as Synthetic Intermediates. II. Syntheses of Homoallyl Ethers from Allylsilanes and Acetals Promoted by Titanium Tetrachloride. Chem. Lett. 1976, 5, 941–942.
- 7 Kira, M.; Kobayashi, M.; Sakurai, H. Regiospecific and Highly Stereoselective Allylation of Aldehydes with Allyltrifluorosilane Activated by Fluoride Ions. Tetrahedron Lett. 1987, 28, 4081–4084.
- 8 Sparks, M. A.; Panek, J. S. Claisen Rearrangements of Enantiomerically Pure C3-(acyloxy)-(E)-vinylsilanes. J. Org. Chem. 1991, 56, 3431–3438.
- 9 Panek, J. S.; Clark, T. D. Ireland-Claisen Rearrangements of Chiral (Z)-vinylsilanes. Highly Diastereoselective Synthesis of anti-α- lkoxy-β-(dimethylphenylsilyl)-(E)-hex-4-enoates. J. Org. Chem. 1992, 57, 4323–4326.
- 10 Suginome, M.; Matsumoto, A.; Ito, Y. New Synthesis of (E)-Allylsilanes with High Enantiopurity via Diastereoselective Intramolecular Bis-Silylation of Chiral Allylic Alcohols. J. Am. Chem. Soc. 1996, 118, 3061–3062.
- 11
Shimizu, M.; Kurahashi, T.; Hiyama, T. Novel Synthesis of 2,3-Bisboryl-1,3-dienes from 1-Bromo-1-lithioethene and 1,1-Bisborylalkenes. Synlett 2001, 2001, 1006–1008.
10.1055/s-2001-14647 Google Scholar
- 12 Gerdin, M.; Moberg, C. Enantioselective Platinum-Catalyzed Silicon-Boron Addition to 1,3-Cyclohexadiene. Adv. Synth. Catal. 2005, 347, 749−753.
- 13 Bourque, L. E.; Cleary, P. A.; Woerpel, K. A. Metal-Catalyzed Silylene Insertions of Allylic Ethers: Stereoselective Formation of Chiral Allylic Silanes. J. Am. Chem. Soc. 2007, 129, 12602–12603.
- 14 Kacprzynski, M. A.; May, T. L.; Kazane, S. A.; Hoveyda, A. H. Enantioselective Synthesis of Allylsilanes Bearing Tertiary and Quaternary Si-Substituted Carbons through Cu-Catalyzed Allylic Alkylations with Alkylzinc and Arylzinc Reagents. Angew. Chem. Int. Ed. 2007, 46, 4554–4558.
- 15 Shintani, R.; Ichikawa, Y.; Hayashi, T.; Chen, J.; Nakao, Y.; Hiyama, T. Catalytic Asymmetric Synthesis of Allylsilanes through Rhodium/ Chiral Diene-Catalyzed 1,4-Addition of Alkenyl[2-(hydroxymethyl)phenyl]dimethylsilanes. Org. Lett. 2007, 9, 4643–4645.
- 16 Gao, F.; McGrath, K. P.; Lee, Y.; Hoveyda, A. H. Synthesis of Quaternary Carbon Stereogenic Centers through Enantioselective Cu-Catalyzed Allylic Substitutions with Vinylaluminum Reagents. J. Am. Chem. Soc. 2010, 132, 14315–14320.
- 17 Binanzer, M.; Fang, G. Y.; Aggarwal, V. K. Asymmetric Synthesis of Allylsilanes by the Borylation of Lithiated Carbamates: Formal Total Synthesis of (–)-Decarestrictine D. Angew. Chem. Int. Ed. 2010, 49, 4264–4268.
- 18 Aggarwal, V. K.; Binanzer, M.; de Ceglie, M. C.; Gallanti, M.; Glasspoole, B. W.; Kendrick, S. J. F.; Sonawane, R. P.; Vázquez- Romero, A.; Webster, M. P. Asymmetric Synthesis of Tertiary and Quaternary Allyl- and Crotylsilanes via the Borylation of Lithiated Carbamates. Org. Lett. 2011, 13, 1490–1493.
- 19 Nagao, K.; Yokobori, U.; Makida, Y.; Ohmiya, H.; Sawamura, M. Reversible 1,3-anti/syn-Stereochemical Courses in Copper-Catalyzed γ-Selective Allyl-Alkyl Coupling between Chiral Allylic Phosphates and Alkylboranes. J. Am. Chem. Soc. 2012, 134, 8982–8987.
- 20 Shido, Y.; Yoshida, M.; Tanabe, M.; Ohmiya, H.; Sawamura, M. Copper-Catalyzed Enantioselective Allylic Substitution with Alkylboranes. J. Am. Chem. Soc. 2012, 134, 18573–18576.
- 21 Yasuda, Y.; Nagao, K.; Shido, Y.; Mori, S.; Ohmiya, H.; Sawamura, M. Copper-Catalyzed γ-Selective and Stereospecific Allylic Cross-Coupling with Secondary Alkylboranes. Chem.-Eur. J. 2015, 21, 9666–9970.
- 22 Davies, H. M. L.; Hansen, T.; Rutberg, J.; Bruzinski, P. R. Rhodium(II) (S)-N-(arylsulfonyl)prolinate Catalyzed Asymmetric Insertions of Vinyl- and Phenylcarbenoids into the Si−H Bond. Tetrahedron Lett. 1997, 38, 1741–1744.
- 23 Hayashi, T.; Han, J. W.; Takeda, A.; Tang, J.; Nohmi, K.; Mukaide, K.; Tsuji, H.; Uozumi, Y. Modification of Chiral Monodentate Phosphine Ligands (MOP) for Palladium-Catalyzed Asymmetric Hydrosilylation of Cyclic 1,3-Dienes. Adv. Synth. Catal. 2001, 343, 279–283.
- 24 Suginome, M.; Ohmura, T.; Miyake, Y.; Mitani, S.; Ito, Y.; Murakami, M. Enantioface-Selective Palladium-Catalyzed Silaboration of Allenes via Double Asymmetric Induction. J. Am. Chem. Soc. 2003, 125, 11174–11175.
- 25 Ohmura, T.; Taniguchi, H.; Suginome, M. Palladium-Catalyzed Asymmetric Silaboration of Allenes. J. Am. Chem. Soc. 2006, 128, 13682–13683.
- 26 Ohmura, T.; Suginome, M. Asymmetric Silaboration of Terminal Allenes Bearing α-Stereogenic Centers: Stereoselection Based on “Reagent Control”. Org. Lett. 2006, 8, 2503–2506.
- 27 Schmidtmann, E. S.; Oestreich, M. Mechanistic Insight into Copper- Catalysed Allylic Substitutions with bis(triorganosilyl) Zincs. Enantiospecific Preparation of α-chiral Silanes. Chem. Commun. 2006, 3643–3645.
- 28 Wu, J.; Chen, Y.; Panek, J. S. Vinylogous Aldol Products from Chiral Crotylsilanes Obtained by Enantioselective Rh(II) and Cu(I) Carbenoid Si−H Insertion. Org. Lett. 2010, 12, 2112–2115.
- 29 Saito, N.; Kobayashi, A.; Sato, Y. Nickel-Catalyzed Enantio- and Diastereoselective Three-Component Coupling of 1,3-Dienes, Aldehydes, and a Silylborane Leading to α-Chiral Allylsilanes. Angew. Chem. Int. Ed. 2012, 51, 1228–1231.
- 30 Lee, K.-S.; Wu, H.; Haeffner, F.; Hoveyda, A. H. NHC–Cu-Catalyzed Silyl Conjugate Additions to Acyclic and Cyclic Dienones and Dienoates. Efficient Site-, Diastereo- and Enantioselective Synthesis of Carbonyl- Containing Allylsilanes. Organometallics 2012, 31, 7823–7826.
- 31 Delvos, L. B.; Vyas, D. J.; Oestreich, M. Asymmetric Synthesis of α-Chiral Allylic Silanes by Enantioconvergent γ-Selective Copper(I)- Catalyzed Allylic Silylation. Angew. Chem. Int. Ed. 2013, 52, 4650–4653.
- 32 Hensel, A.; Oestreich, M. Asymmetric Catalysis with Silicon-Based Cuprates: Enantio- and Regioselective Allylic Substitution of Linear Precursors. Chem.-Eur. J. 2015, 21, 9062–9065.
- 33 Hofstra, J. L.; Cherney, A. H.; Ordner, C. M.; Reisman, S. E. Synthesis of Enantioenriched Allylic Silanes via Nickel-Catalyzed Reductive Cross-Coupling. J. Am. Chem. Soc. 2018, 140, 139–142.
- 34 Da, B.-C.; Liang, Q.-J.; Luo, Y.-C.; Ahmad, T.; Xu, Y.-H.; Loh, T.-P. Copper-Catalyzed Stereo- and Enantioselective 1,4-Protosilylation of α,β-Unsaturated Ketimines to Synthesize Functionalized Allylsilanes. ACS Catal. 2018, 8, 6239–6245.
- 35 Zheng, K.; Liu, X.; Feng, X. Recent Advances in Metal-Catalyzed Asymmetric 1,4-Conjugate Addition (ACA) of Nonorganometallic Nucleophiles. Chem. Rev. 2018, 118, 7586–7656.
- 36 Oestreich, M.; Hartmann, E.; Mewald, M. Activation of the Si–B Interelement Bond: Mechanism, Catalysis, and Synthesis. Chem. Rev. 2013, 113, 402–441.
- 37 Feng, J.-J.; Mao, W.; Zhang, L.; Oestreich, M. Activation of the Si–B interelement bond related to catalysis. Chem. Soc. Rev. 2021, 50, 2010–2073.
- 38 Walter, C.; Auer, G.; Oestreich, M. Rhodium-Catalyzed Enantioselective Conjugate Silyl Transfer: 1,4-Addition of Silyl Boronic Esters to Cyclic Enones and Lactones. Angew. Chem. Int. Ed. 2006, 45, 5675–5677.
- 39 Walter, C.; Fröhlich, R.; Oestreich, M. Rhodium(I)-Catalyzed Enantioselective 1,4-addition of Nucleophilic Silicon. Tetrahedron 2009, 65, 5513–5520.
- 40 Walter, C.; Oestreich, M. Catalytic Asymmetric C–Si Bond Formation to Acyclic α,β-Unsaturated Acceptors by Rh(I)-Catalyzed Conjugate Silyl Transfer Using a Si–B Linkage. Angew. Chem. Int. Ed. 2008, 47, 3818–3820.
- 41 Hartmann E.; Oestreich, M. Asymmetric Conjugate Silyl Transfer in Iterative Catalytic Sequences: Synthesis of the C7–C16 Fragment of (+)-Neopeltolide. Angew. Chem. Int. Ed. 2010, 49, 6195–6198.
- 42 Hartmann, E.; Oestreich, M. Two-Directional Desymmetrization by Double 1,4-Addition of Silicon and Boron Nucleophiles. Org. Lett. 2012, 14, 2406–2409.
- 43 Mao, W.; Xue, W.; Irran, E.; Oestreich, M. Copper-Catalyzed Regio- and Enantioselective Addition of Silicon Grignard Reagents to Alkenes Activated by Azaaryl Groups. Angew. Chem. Int. Ed. 2019, 58, 10723–10726.
- 44 Xue, W.; Oestreich, M. Beyond Carbon: Enantioselective and Enantiospecific Reactions with Catalytically Generated Boryl- and Silylcopper Intermediates. ACS Cent. Sci. 2020, 6, 1070–1081.
- 45 Lee, K.-S.; Hoveyda, A. H. Enantioselective Conjugate Silyl Additions to Cyclic and Acyclic Unsaturated Carbonyls Catalyzed by Cu Complexes of Chiral N-Heterocyclic Carbenes. J. Am. Chem. Soc. 2010, 132, 2898–2900.
- 46 Pace, V.; Rae, J. P.; Procter, D. J. Cu(I)–NHC Catalyzed Asymmetric Silyl Transfer to Unsaturated Lactams and Amides. Org. Lett. 2014, 16, 476–479.
- 47 Pace, V.; Rae, J. P.; Harb, H. Y.; Procter, D. J. NHC–Cu(I) Catalysed Asymmetric Conjugate Silyl Transfer to Unsaturated lactones: Application in Kinetic Resolution. Chem. Commun. 2013, 49, 5150–5152.
- 48O′Brien, J. M.; Hoveyda, A. H. Metal-Free Catalytic C–Si Bond Formation in an Aqueous Medium. Enantioselective NHC-Catalyzed Silyl Conjugate Additions to Cyclic and Acyclic α,β-Unsaturated Carbonyls. J. Am. Chem. Soc. 2011, 133, 7712–7715.
- 49 Wu, H.; Garcia, J. M.; Haeffner, F.; Radomkit, S.; Zhugralin, A. R.; Hoveyda, A. H. Mechanism of NHC-Catalyzed Conjugate Additions of Diboron and Borosilane Reagents to α,β-Unsaturated Carbonyl Compounds. J. Am. Chem. Soc. 2015, 137, 10585–10602.
- 50 Ibrahem, I.; Santoro, S.; Himo, F.; Córdova, A. Enantioselective Conjugate Silyl Additions to α,β-Unsaturated Aldehydes Catalyzed by Combination of Transition Metal and Chiral Amine Catalysts. Adv. Synth. Catal. 2011, 353, 245–252.
- 51 Kitanosono, T.; Zhu, L.; Liu, C.; Xu, P.; Kobayashi, S. An Insoluble Copper(II) Acetylacetonate–Chiral Bipyridine Complex that Catalyzes Asymmetric Silyl Conjugate Addition in Water. J. Am. Chem. Soc. 2015, 137, 15422–15425.
- 52 Clavier, H.; Coutable, l.; Guillemin, J.-C.; Mauduit, M. New Bidentate Alkoxy-NHC Ligands for Enantioselective Copper-Catalysed Conjugate Addition. Tetrahedron: Asymmetry 2005, 16, 921–924.
- 53 Clavier, H.; Coutable, L.; Toupet, L.; Guillemin, J.-C.; Mauduit, M. Design and Synthesis of new Bidentate Alkoxy-NHC Ligands for Enantioselective Copper-Catalyzed Conjugate Addition. J. Organomet. Chem. 2005, 690, 5237–5254.
- 54 Wencel, J.; Mauduit, M.; Hénon, H.; Kehrli, S.; Alexakis, A. Chiral, Chelating, Hydroxyakyl and Hydroxyaryl N Heterocyclic Carbenes; Design, Synthesis, and Application in Copper–Catalyzed Asymmetric Conjugate Addition. Aldrichim. Acta 2009, 42, 43–50.
- 55 Jahier-Diallo, C.; Morin, M. S. T.; Queval, P.; Rouen, M.; Artur, I.; Querard, P.; Toupet, L.; Crévisy, C.; Baslé, O.; Mauduit, M. Multicomponent Synthesis of Chiral Bidentate Unsymmetrical Unsaturated N-Heterocyclic Carbenes: Copper–Catalyzed Asymmetric C–C Bond Formation. Chem.-Eur. J. 2015, 21, 993–997.
- 56 Brown, M. K.; May, T. L.; Baxter, C. A.; Hoveyda, A. H. All-Carbon Quaternary Stereogenic Centers by Enantioselective Cu-Catalyzed Conjugate Additions Promoted by a Chiral N-Heterocyclic Carbene. Angew. Chem. Int. Ed. 2007, 46, 1097–1100.
- 57 Park, J. K.; Lackey, H. H.; Rexford, M. D.; Kovnir, K.; Shatruk, M.; McQuade, D. T. A Chiral 6-Membered N-Heterocyclic Carbene Copper(I) Complex That Induces High Stereoselectivity. Org. Lett. 2010, 12, 5008–5011.
- 58 Park, J. K.; McQuade, D. T. Iterative Asymmetric Allylic Substitutions: syn- and anti- 1,2-Diols through Catalyst Control. Angew. Chem. Int. Ed. 2012, 51, 2717–2721.
- 59 Park, J. K.; McQuade, D. T. Chiral 6-NHC Ligand and Copper Complex: Properties, Application, and Mechanism. Synthesis 2012, 44, 1485–1490.
- 60 Delvos, L. B.; Hensel, A.; Oestreich, M. McQuade's Six-Membered NHC-Copper(I) Complexes for Catalytic AsymmetricSilyl Transfer. Synthesis 2014, 46, 2957–2964.




