Phosphorus Ligands from the Zhang Lab: Design, Asymmetric Hydrogenation, and Industrial Applications
Feng Wan
State Key Laboratory of Bio-Organic & Natural Products Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 China
Search for more papers by this authorCorresponding Author
Wenjun Tang
State Key Laboratory of Bio-Organic & Natural Products Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 China
School of Chemistry and Materials Science, Hangzhou Institute for Advanced Study, University of Chinese Academy of Sciences, 1 Sub-lane Xiangshan, Hangzhou, Zhejiang, 310024 China
E-mail: [email protected]Search for more papers by this authorFeng Wan
State Key Laboratory of Bio-Organic & Natural Products Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 China
Search for more papers by this authorCorresponding Author
Wenjun Tang
State Key Laboratory of Bio-Organic & Natural Products Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 China
School of Chemistry and Materials Science, Hangzhou Institute for Advanced Study, University of Chinese Academy of Sciences, 1 Sub-lane Xiangshan, Hangzhou, Zhejiang, 310024 China
E-mail: [email protected]Search for more papers by this authorAbstract
Chiral phosphorus ligands have played a crucial role for the recent advances in asymmetric catalysis. This review summarizes chiral phosphorus ligands developed from the Laboratory of Professor Xumu Zhang in the latest 25 years. A number of iconic phosphorus ligands including bisphosphorus ligands with rigid chiral backbone such as BICP, PennPhos, TunePhos, and f-Binaphane, P-chiral bisphosphorus ligands TangPhos, Binapine, and DuanPhos, phosphine-phosphoramidte ligand YanPhos, noncovalent interaction-assisted ferrocenyl phosphorus ligand ZhaoPhos and WudaPhos, and tridentate ferrocenyl phosphorus ligands f-amphox are introduced, and their applications in asymmetric hydrogenation are emphasized.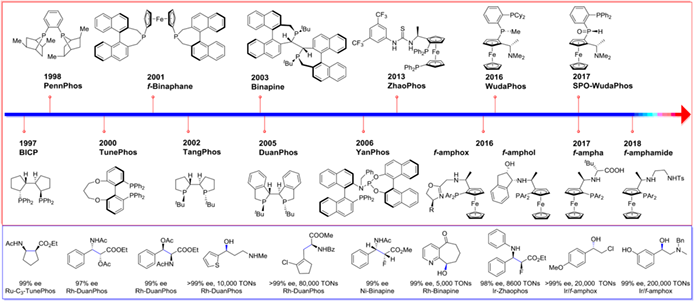
References
- 1 Tang, W.; Zhang, X. New Chiral Phosphorus Ligands for Enatioselective Hydrogenation. Chem. Rev. 2003, 103, 3029–3070.
- 2 Kagan, H. B.; Dang, T.-P. Asymmetric Catalytic Reduction with Transition Metal Complexes. I. A Catalytic System of Rhodium(I) with (–)-2,3-O-Isopropylidene-2,3-Dihydroxy-1,4-Bis(Diphenylphosphino) Butane, a New Chiral Diphosphine. J. Am. Chem. Soc. 1972, 94, 6429–6433.
- 3(a) Vineyard, B. D.; Knowles, W. S.; Sabacky, M. J.; Bachman, G. L.; Weinkauff, O. J. Asymmetric Hydrogenation. Rhodium Chiral Bisphosphine Catalyst. J. Am. Chem. Soc. 1977, 99, 5946–5952; (b) Knowles, W. S. Asymmetric Hydrogenation. Acc. Chem. Res. 1983, 16, 106–112; (c) Knowles, W. S. Asymmetric Hydrogenations (Nobel Lecture). Angew. Chem. Int. Ed. 2002, 41, 1998–2007.
- 4(a) Miyashita, A.; Yasuda, A.; Takaya, H.; Toriumi, K.; Ito, T.; Souchi, T.; Noyori, R. Synthesis of 2,2’-Bis(diphenylphosphino)-1,1’-binaphthyl (BINAP), an Atropisomeric Chiral Bis(triaryl)phosphine, and Its Use in the Rhodium(I)-Catalyzed Asymmetric Hydrogenation of R-(Acylamino)acrylic Acids. J. Am. Chem. Soc. 1980, 102, 7932–7934;
(b) Noyori, R.; Ohkuma, T. Asymmetric Catalysis by Architectural and Functional Molecular Engineering: Practical Chemo- and Stereoselective Hydrogenation of Ketones. Angew. Chem. Int. Ed. 2001, 40, 40–73;
(c) Noyori, R. Asymmetric Catalysis: Science and Opportunities (Nobel Lecture). Angew. Chem. Int. Ed. 2002, 41, 2008–2022.
10.1002/1521-3773(20020617)41:12<2008::AID-ANIE2008>3.0.CO;2-4 CAS PubMed Web of Science® Google Scholar
- 5 Schmit, R.; Foricher, J.; Cereghetti, M.; Schönholzer, P. Axially Dissymmetric Diphosphines in the Biphenyl Series: Synthesis of (6,6’-Dimethoxybiphenyl-2,2’-diyl)bis (diphenylphosphine) (‘MeO- BIPHEP’) and Analogues via an ortho-Lithiation/Iodination Ullmann- Reaction Approach. Helv. Chim. Acta 1991, 74, 370–381.
- 6(a) Burk, M. J. C2-Symmetric Bis(phospholanes) and Their Use in Highly Enantioselective Hydrogenation Reactions. J. Am. Chem. Soc. 1991, 113, 8518–8519; (b) Burk, M. J.; Feaster, J. E.; Nugent, W. A.; Harlow, R. L. Preparation and Use of C2-Symmetric Bis-(phospholanes): Production of R-Amino Acid Derivatives via Highly Enantioselective Hydrogenation Reactions. J. Am. Chem. Soc. 1993, 115, 10125–10138; (c) Burk, M. J. Modular Phospholane Ligands in Asymmetric Catalysis. Acc. Chem. Res. 2000, 33, 363–372.
- 7(a) Zhao, Q.; Chen, C.; Wen, J.; Dong, X. Q.; Zhang, X. Noncovalent Interaction-Assisted Ferrocenyl Phosphine Ligands in Asymmetric Catalysis. Acc. Chem. Res. 2020, 53, 1905–1921;
(b) Tang, W. J.; Zhang, X. M. New Chiral Phosphorus Ligands for Enantioselective Hydrogenation. Chem. Rev. 2003, 103, 3029–3069;
(c) Zhang, W.; Chi, Y.; Zhang, X. Developing Chiral Ligands for Asymmetric Hydrogenation. Acc. Chem. Res. 2007, 40, 1278–1290;
(d) Li, W.; Zhang, X. Asymmetric Hydrogenation of Imines. In Stereoselective Formation of Amines. Topics in Current Chemistry-Series, Vol. 343, Eds.: Li, W.; Zhang, X., Springer, 2014, pp. 103–144;
(e) Chen, C.; Lu, H.; Zhang, X. Progresses in Rh-Catalyzed Linear Hydroformylation. Petrochem. Technol. 2015, 44, 1149–1156;
(f) Dong, X.-Q.; Zhao, Q.; Li, P.; Chen, C.; Zhang, X. Metalorganocatalysis: Cooperating Transition-Metal Catalysis and Organocatalysis through a Covalent Bond. Org. Chem. Front. 2015, 2, 1425–1431;
(g) Chen, C.; Dong, X.-Q.; Zhang, X. Recent Progress in Rhodium-Catalyzed Hydroaminomethylation. Org. Chem. Front. 2016, 3, 1359–1370;
(h) Li, P.; Hu, X.; Dong, X.-Q.; Zhang, X. Recent Advances in Dynamic Kinetic Resolution by Chiral Bifunctional (Thio)urea- and Squaramide-Based Organocatalysts. Molecules 2016, 21, 1327;
(i) Li, S.; Li, Z.; You, C.; Lv, H.; Zhang, X. Recent Advances in Asymmetric Hydroformylation. Chin. J. Org. Chem. 2019, 39, 1568–1582;
(j) Liu, Y.; Dong, X.-Q.; Zhang, X. Recent Advances of Nickel-Catalyzed Homogeneous Asymmetric Hydrogenation. Chin. J. Org. Chem. 2020, 40, 1096–1104;
(k) Li, K.; Nie, M.; Tang, W. Synthesis of α-Tertiary Allylsilanes by Palladium-Catalyzed Hydrosilylation of 1,1-Disubstituted Allenes. Green Syn. Catal. 2020, 1, 171–174.
10.1016/j.gresc.2020.08.003 Google Scholar
- 8(a) Zhu, G.; Cao, P.; Jiang, Q.; Zhang, X. High Enantioselective Rh-Catalyzed Hydrogenations with a New Chiral 1,4-Bisphosphine Containing a Cyclic Backbone. J. Am. Chem. Soc. 1997, 119, 1799–1800; (b) Zhu, G.; Zhang, X. Asymmetric Rh-Catalyzed Hydrogenation of Enamides with a Chiral 1,4-Bisphosphine Bearing Diphenyl-phosphino Groups. J. Org. Chem. 1998, 63, 9590–9593; (c) Zhu, G.; Casalnuovo, A. L.; Zhang, X. Practical Syntheses of β-Amino Alcohols via Asymmetric Catalytic Hydrogenation. J. Org. Chem. 1998, 63, 8100–8101; (d) Zhu, G.; Zhang, X. Additive Effects in Ir-BICP Catalyzed Asymmetric Hydrogenation of Imines. Tetrahedron Asymmetry 1998, 9, 2415–2418; (e) Zhu, G.; Chen, Z.; Zhang, X. Highly Efficient Asymmetric Synthesis of β-Amino Acid Derivatives via Rhodium-Catalyzed Hydrogenation of β-(Acylamino)Acrylates. J. Org. Chem. 1999, 64, 6907–6910; (f) Cao, P.; Zhang, X. Ru-BICP-Catalyzed Asymmetric Hydrogenation of Aromatic Ketones. J. Org. Chem. 1999, 64, 2127–2129; (g) Li, W.; Zhang, X. Synthesis of 3,4-O-Isopropylidene-(3S,4S)- dihydroxy-(2R,5R)-bis(diphenylphosphino) hexane and Its Application in Rh-Catalyzed Highly Enantioselective Hydrogenation of Enamides. J. Org. Chem. 2000, 65, 5871–5874.
- 9(a) Jiang, Q.; Jiang, Y.; Xiao, D.; Cao, P.; Zhang, X. High Enantioselective Hydrogenation of Simple Ketones Catalyzed by a Rh-PennPhos Complex. Angew. Chem. Int. Ed. 1998, 37, 1100–1103;
10.1002/(SICI)1521-3773(19980504)37:8<1100::AID-ANIE1100>3.0.CO;2-3 CAS PubMed Web of Science® Google Scholar(b) Zhang, Z.; Zhu, G.; Jiang, Q.; Xiao, D.; Zhang, X. Highly Enantioselective Hydrogenation of Cyclic Enamides Catalyzed by a Rh-PennPhos Catalyst. J. Org. Chem. 1999, 64, 1774–1775; (c) Jiang, Q.; Xiao, D.; Zhang, Z.; Cao, P.; Zhang, X. Highly Enantioselective Hydrogenation of Cyclic Enol Acetates Catalyzed by a Rh-PennPhos Complex. Angew. Chem. Int. Ed. 1999, 38, 516–518.10.1002/(SICI)1521-3773(19990215)38:4<516::AID-ANIE516>3.0.CO;2-B CAS PubMed Web of Science® Google Scholar
- 10 Tang, W.; Wu, S.; Zhang, X. Enantioselective Hydrogenation of Tetrasubstituted Olefins of Cyclic β-(Acylamino)acrylates. J. Am. Chem. Soc. 2003, 125, 9570–9571.
- 11 Wu, S.; Wang, W.; Tang, W.; Lin, M.; Zhang, X. Highly Enantioselective Hydrogenation of Enol Acetates Catalyzed by Ru−TunaPhos Complexes. Org. Lett. 2002, 4, 4495–4497.
- 12 Lei, A.; Wu, S.; He, M.; Zhang, X. Highly Enantioselective Asymmetric Hydrogenation of α-Phthalimide Ketone: An Efficient Entry to Enantiomerically Pure Amino Alcohols. J. Am. Chem. Soc. 2004, 126, 1626–1627.
- 13 Sun, X.; Zhou, L.; Li, W.; Zhang, X. Convenient Divergent Strategy for the Synthesis of TunePhos-Type Chiral Diphosphine Ligands and Their Applications in Highly Enantioselective Ru-Catalyzed Hydrogenations. J. Org. Chem. 2008, 73, 1143–1146.
- 14 Li, W.; Sun, X.; Zhou, L.; Hou, G.; Yu, S.; Zhang, X. Highly Efficient and Highly Enantioselective Asymmetric Hydrogenation of Ketones with TunesPhos/1,2-Diamine-Ruthenium(II) Complexes. J. Org. Chem. 2009, 74, 1397–1399.
- 15 Zhang, Z.; Qian, H.; Longmire, J.; Zhang, X. Synthesis of Chiral Bisphosphines with Tunable Bite Angles and Their Applications in Asymmetric Hydrogenation of β-Ketoesters. J. Org. Chem. 2000, 65, 6223–6226.
- 16(a) Xiao, D.; Zhang, X. Highly Enantioselective Hydrogenation of Acyclic Imines Catalyzed by Ir-f-Binaphane Complexes. Angew. Chem. Int. Ed. 2001, 40, 3425–3428;
10.1002/1521-3773(20010917)40:18<3425::AID-ANIE3425>3.0.CO;2-O CAS PubMed Web of Science® Google Scholar(b) Chi, Y.; Zhou, Y.-G.; Zhang, X. Highly Enantioselective Reductive Amination of Simple Aryl Ketones Catalyzed by Ir-f-Binaphane in the Presence of Titanium(IV) Isopropoxide and Iodine. J. Org. Chem. 2003, 68, 4120–4122; (c) Chang, M.; Li, W.; Hou, G.; Zhang, X. Iridium-Catalyzed Enantioselective Hydrogenation of Cyclic Imines. Adv. Synth. Catal. 2010, 352, 3121–3125; (d) Chang, M.; Li, W.; Zhang, X. A Highly Efficient and Enantioselective Access to Tetrahydroisoquinoline Alkaloids: Asymmetric Hydrogenation with an Iridium Catalyst. Angew. Chem. Int. Ed. 2011, 50, 10679–10681; (e) Xiao, D.; Zhang, Z.; Zhang, X. Synthesis of a Novel Chiral Binaphthyl Phospholane and Its Application in the Highly Enantioselective Hydrogenation of Enamides. Org. Lett. 1999, 1, 1679–1681.
- 17(a) Tang, W.; Zhang, X. A Chiral 1,2-Bisphospholane Ligand with a Novel Structural Motif: Applications in Highly Enantioselective Rh-Catalyzed Hydrogenations. Angew. Chem. Int. Ed. 2002, 41, 1612–1614;
10.1002/1521-3773(20020503)41:9<1612::AID-ANIE1612>3.0.CO;2-H CAS PubMed Web of Science® Google Scholar(b) Tang, W.; Zhang, X. Highly Efficient Synthesis of Chiral β-Amino Acid Derivatives via Asymmetric Hydrogenation. Org. Lett. 2002, 4, 4159–4161; (c) Tang, W.; Liu, D.; Zhang, X. Asymmetric Hydrogenation of Itaconic Acid and Enol Acetate Derivatives with the Rh-TangPhos Catalyst. Org. Lett. 2003, 5, 205–207; (d) Dai, Q.; Yang, W.; Zhang, X. Efficient Rhodium-Catalyzed Asymmetric Hydrogenation for the Synthesis of a New Class of N-Aryl β-Amino Acid Derivatives. Org. Lett. 2005, 7, 5343–5345; (e) Yang, Q.; Gao, W.; Deng, J.; Zhang, X. Highly Enantioselective Hydrogenation of N-Phthaloyl Enamides. Tetrahedron Lett. 2006, 47, 821–823; (f) Wang, C.; Tao, H.; Zhang, X. Highly Enantioselective Ru-Catalyzed Hydrogenation of β-Keto Esters Using Electron-Donating Bis(trialkylphosphine) Ligand- TangPhos. Tetrahedron Lett. 2006, 47, 1901–1903; (g) Yang, Q.; Shang, G.; Gao, W.; Deng, J.; Zhang, X. A Highly Enantioselective, Pd-TangPhos-Catalyzed Hydrogenation of N-Tosylimines. Angew. Chem. Int. Ed. 2006, 45, 3832–3835; (h) Shang, G.; Yang, Q.; Zhang, X. Rh-Catalyzed Asymmetric Hydrogenation of β-Aryl Imino Esters: An Efficient Enantioselective Synthesis of Aryl Glycine Derivatives. Angew. Chem. Int. Ed. 2006, 45, 6360–6362; (i) Lei, A.; Chen, M.; He, M.; Zhang, X. Asymmetric Hydrogenation of Pyridines: Enantioselective Synthesis of Nipecotic Acid Derivatives. Eur. J. Org. Chem. 2006, 4343–4347.
- 18(a) Liu, D.; Zhang, X. Practical P-Chiral Phosphane Ligand for Rh-Catalyzed Asymmetric Hydrogenation. Eur. J. Org. Chem. 2005, 646–649; (b) Liu, D.; Gao, W.; Wang, C.; Zhang, X. Practical Synthesis of Enantiopure γ-Amino Alcohols by Rhodium-Catalyzed Asymmetric Hydrogenation of γ-Secondary-Amino Ketones. Angew. Chem. Int. Ed. 2005, 44, 1687–1689; (c) Sun, X.; Zhou, L.; Wang, C.-J.; Zhang, X. Rh-Catalyzed Highly Enantioselective Synthesis of β-Methylcinnamic Acids. Angew. Chem. Int. Ed. 2007, 46, 2623–2626; (d) Geng, H.; Zhang, W.; Chen, J.; Hou, G.; Zhou, L.; Zou, Y.; Wu, W.; Zhang, X. Rhodium-Catalyzed Enantioselective and DiastereoselectiveHydrogenation of b-Ketoenamides: Efficient Access to anti 1,3-Amino Alcohols. Angew. Chem. Int. Ed. 2009, 48, 6052–6054; (e) Lou, Y.; Wang, J.; Gong, G.; Guan, F.; Lu, J.; Wen, J.; Zhang, X. Catalytic Asymmetric Hydrogenation of (Z)-α-Dehydroamido Boronate Esters: Direct Route to Alkyl-Substituted α-Amidoboronic Esters. Chem. Sci. 2020, 11, 851–855; (f) Wang, Q.; Huang, W.; Yuan, H.; Cai, Q.; Chen, L.; Lv, H.; Zhang, X. Rhodium-Catalyzed Enantioselective Hydrogenation of Tetrasubstituted α-Acetoxy β-Enamido Esters: A New Approach to Chiral α-Hydroxyl-β-amino Acid Derivatives. J. Am. Chem. Soc. 2014, 136, 16120–16123; (g) Guan, Y.; Gao, M.; Deng, X.; Lv, H.; Zhang, X. Rhodium-Catalyzed Asymmetric Hydrogenation of Tetrasubstituted β-Acetoxy-α-Enamido Esters and Efficient Synthesis of Droxidopa. Chem. Commun. 2017, 53, 8136–8139; (h) Wang, Q.; Gao, W.; Lv, H.; Zhang, X. Enantioselective Synthesis of β-Substituted Chiral Allylic Amines via Rh-Catalyzed Asymmetric Hydrogenation. Chem. Commun. 2016, 52, 11850–11853; (i) Zhang, J.; Lu, W.; Lv, H.; Zhang, X. Highly Efficient Synthesis of Chiral α-CF3 Amines via Rh-Catalyzed Asymmetric Hydrogenation. Org. Lett. 2015, 17, 1154–1156; (j) Ji, J.; Chen, C.; Cai, J.; Wang, X.; Zhang, K.; Shi, L.; Lv, H.; Zhang, X. Highly Enantioselective Synthesis of Non-Natural Aliphatic α-Amino Acids via Asymmetric Hydrogenation. Org. Biomol. Chem. 2015, 13, 7624–7627; (k) Zhou, M.; Liu, T.; Cao, M.; Xue, Z.; Lv, H.; Zhang, X. Highly Enantioselective Synthesis of Chiral Cyclic Allylic Amines via Rh-Catalyzed Asymmetric Hydrogenation. Org. Lett. 2014, 16, 3484–3487; (l) Yang, H.; Wang, E.; Yang, P.; Lv, H.; Zhang, X. Pyridine-Directed Asymmetric Hydrogenation of 1,1-Diarylalkenes. Org. Lett. 2017, 19, 5062–5065; (m) Gao, W.; Wang, Q.; Xie, Y.; Lv, H.; Zhang, X. Rhodium-Catalyzed Asymmetric Hydrogenation of α-Dehydroamino Ketones: A General Approach to Chiral α-amino Ketones. Chem. Asian J. 2016, 11, 231–233; (n) Li, W.; Hou, G.; Chang, M.; Zhang, X. Highly Efficient and Enantioselective Iridium-Catalyzed Asymmetric Hydrogenation of N-Arylimines. Adv. Synth. Catal. 2009, 351, 3123–3127.
- 19 Wu, S.; Yu, B.; Wang, Y.; Zhu, J. Process and Intermediates for the Preparation of N-Acylated-4-Aryl Beta-Amino Acid Derivatives. CN 102271504 B, 2014.
- 20(a) Tang, W.; Wang, W.; Chi, Y.; Zhang, X. A Bisphosphepin Ligand with Stereogenic Phosphorus Centers for the Practical Synthesis of α-Aryl-β-amino Acids by Asymmetric Hydrogenation. Angew. Chem. Int. Ed. 2003, 42, 3509–3511; (b) Guan, Y.; Han, Z.; Li, X.; You, C.; Tan, X.; Lv, H.; Zhang, X. A Cheap Metal for a Challenging Task: Nickel Catalyzed Highly Diastereo- and Enantioselective Hydrogenation of Tetrasubstituted Fluorinated Enamides. Chem. Sci. 2019, 10, 252–256; (c) Yang, H.; Huo, N.; Yang, P.; Pei, H.; Lv, H.; Zhang, X. Rhodium Catalyzed Asymmetric Hydrogenation of 2-Pyridine Ketones. Org. Lett. 2015, 17, 4144−4147; (d) Li, X.; You, C.; Yang, H.; Che, J.; Chen, P.; Yang, Y.; Lv, H.; Zhang, X. Rhodium-Catalyzed Asymmetric Hydrogenation of Tetrasubstituted Cyclic Enamides: Efficient Access to Chiral Cycloalkylamine Derivatives. Adv. Synth. Catal. 2017, 359, 597–602.
- 21(a) Claver, C.; van Leeuwen, P. W. N. M. Rhodium Catalyzed Hydroformylation, Kluwer Academic Publishers, Dordrecht, 2002; (b) Franke, R.; Selent, D.; Börner, A. Applied Hydroformylation. Chem. Rev. 2012, 112, 5675–5732; (c) Su, K.; Jiang, H.; Zhu, D.; Fu, H.; Zheng, X.; Yuan, M.; Li, R.; Chen, H. Study on 1-Octene Hydroformylation Promoted by Cetyltrihydroxyethyl Ammonium Bromide in Aqueous/ Organic Biphasic Solution. Acta Chim. Sinica 2013, 71, 844–848.
- 22(a) Sakai, N.; Mano, S.; Nozaki, K.; Takaya, H. Highly Enantioselective Hydroformylation of Olefins Catalyzed by New Phosphine Phosphite- Rhodium(I) Complexes. J. Am. Chem. Soc. 1993, 115, 7033–0734; (b) Nozaki, K.; Nanno, T.; Takaya, H. J. Organomet. Chem. 1997, 527, 103; (c) Lambers-Verstappen, M. M. H.; de Vries, J. G. Rhodium-Catalysed Asymmetric Hydroformylation of Unsaturated Nitriles. Adv. Synth. Catal. 2003, 345, 478–482; (d) Tanaka, R.; Nakano, K.; Nozaki, K. Synthesis of α-Heteroarylpropanoic Acid via Asymmetric Hydroformylation Catalyzed by Rh(I)-(R,S)-BINAPHOS and the Subsequent Oxidation. J. Org. Chem. 2007, 72, 8671–8676.
- 23(a) Yan, Y.; Zhang, X. A Hybrid Phosphorus Ligand for Highly Enantioselective Asymmetric Hydroformylation. J. Am. Chem. Soc. 2006, 128, 7198–7202; (b) Zhang, X.; Cao, B.; Yan, Y.; Yu, S.; Ji, B. M.; Zhang, X. Synthesis and Application of Modular Phosphine–Phosphoramidite Ligands in Asymmetric Hydroformylation: Structure–Selectivity Relationship. Chem.-Eur. J. 2010, 16, 871–877; (c) Zhang, X.; Cao, B.; Yu, S.; Zhang, X. Rhodium-Catalyzed Asymmetric Hydroformylation of N-Allylamides: Highly Enantioselective Approach to β-Amino Aldehydes. Angew. Chem. Int. Ed. 2010, 49, 4047–4050; (d) Wei, B.; Chen, C.; You, C.; Lv, H.; Zhang, X. Efficient Synthesis of (S,R)-Bn-Yanphos and Rh/(S, R)-Bn-Yanphos Catalyzed Asymmetric Hydroformylation of Vinyl Heteroarenes. Org. Chem. Front. 2017, 4, 288–291.
- 24 You, C.; Wei, B.; Li, X.; Yang, Y.; Liu, Y.; Lv, H.; Zhang, X. Rhodium- Catalyzed Desymmetrization by Hydroformylation of Cyclopentenes: Synthesis of Chiral Carbocyclic Nucleosides. Angew. Chem. Int. Ed. 2016, 55, 6511–6514.
- 25 You, C.; Li, S.; Li, X.; Lan, J.; Yang, Y.; Chung, L.-W.; Lv, H.; Zhang, X. Design and Application of Hybrid Phosphorus Ligands for Enantioselective Rh-Catalyzed Anti-Markovnikov Hydroformylation of Unfunctionalized 1,1-Disubstituted Alkenes. J. Am. Chem. Soc. 2018, 140, 4977–4981.
- 26 You, C.; Li, X.; Yang, Y.; Yang, Y.-S.; Tan, X.; Li, S.; Wei, B.; Lv, H.; Chung, L.-W.; Zhang, X. Silicon-Oriented Regio- and Enantioselective Rhodium- Catalyzed Hydroformylation. Nat. Commun. 2018, 9, 2045–2053.
- 27 Xu, K.; Zheng, X.; Wang, Z.; Zhang, X. Easily Accessible and Highly Tunable Bisphosphine Ligands for Asymmetric Hydroformylation of Terminal and Internal Alkenes. Chem.-Eur. J. 2014, 20, 4357–4362.
- 28 Zhang, D.; You, C.; Li, X.; Wen, J.; Zhang, X. Asymmetric Linear-Selective Hydroformylation of 1,1-Dialkyl Olefins Assisted by a Steric-Auxiliary Strategy. Org. Lett. 2020, 22, 4523–4526.
- 29 Li, S.; Li, Z.; You, C.; Li, X.; Yang, J.; Lv, H.; Zhang, X. Rhodium-Catalyzed Enantioselective Anti-Markovnikov Hydroformylation of α-Substituted Acryl Acid Derivatives. Org. Lett. 2020, 22, 1108−1112.
- 30(a) You, C.; Li, S.; Li, X.; Lv, H.; Zhang, X. Enantioselective Rh-Catalyzed Anti-Markovnikov Hydroformylation of 1,1-Disubstituted Allylic Alcohols and Amines: An Efficient Route to Chiral Lactones and Lactams. ACS Catal. 2019, 9, 8529−8533; (b) Chen, C.; Jin, S.; Zhang, Z.; Wei, B.; Wang, H.; Zhang, H.; Lv, H.; Zhang, X. Rhodium/Yanphos- Catalyzed Asymmetric Interrupted Intramolecular Hydroaminomethylation of trans-1,2-Disubstituted Alkenes. J. Am. Chem. Soc. 2016, 138, 9017−9020.
- 31(a) Aratani, T.; Gonda, T.; Nozaki, H. Asymmetric Lithiation of Ferrocenes. Tetrahedron Lett. 1969, 10, 2265−2268;
10.1016/S0040-4039(01)88137-5 Google Scholar(b) Marquarding, D.; Klusacek, H.; Gokel, G.; Hoffmann, P.; Ugi, I. Stereoselective Syntheses. VI. Correlation of Central and Planar Chirality in Ferrocene Derivatives. J. Am. Chem. Soc. 1970, 92, 5389−5393
- 32
Hayashi, T.; Yamamoto, K.; Kumada, M. Asymmetric catalytic hydrosilylation of ketones preparation of chiral ferrocenylphosphines as chiral ligands. Tetrahedron Lett. 1974, 15, 4405−4408.
10.1016/S0040-4039(01)92175-6 Google Scholar
- 33(a) Hayashi, T.; Kumada, M. Asymmetric synthesis catalyzed by Transition-Metal Complexes with Functionalized Chiral Ferrocenylphosphine Ligands. Acc. Chem. Res. 1982, 15, 395−401; (b) Ito, Y.; Sawamura, M.; Hayashi, T. Catalytic Asymmetric Aldol Reaction: Reaction of Aldehydes with Isocyanoacetate Catalyzed by a Chiral Ferrocenylphosphine-Gold(I) Complex. J. Am. Chem. Soc. 1986, 108, 6405−6406; (c) Hayashi, T.; Yamamoto, A.; Hagihara, T.; Ito, Y. Modification of Optically Active Ferrocenylphosphine Ligands for Palladium-Catalyzed Asymmetric Allylic Alkylation. Tetrahedron Lett. 1986, 27, 191−194
- 34(a) Chen, W.; McCormack, P. J.; Mohammed, K.; Mbafor, W.; Roberts, S. M.; Whittall, J. Stereoselective Synthesis of FerroceneBased C2-Symmetric Diphosphine Ligands: Application to the Highly Enantioselective Hydrogenation of α-Substituted Cinnamic Acids. Angew. Chem. Int. Ed. 2007, 46, 4141−4144; (b) Chen, W.; Spindler, F.; Pugin, B.; Nettekoven, U. ChenPhos: Highly Modular PStereogenic C1-Symmetric Diphosphine Ligands for the Efficient Asymmetric Hydrogenation of α-Substituted Cinnamic Acids. Angew. Chem. Int. Ed. 2013, 52, 8652−8656.
- 35 Jakab, G.; Schreiner, P. R. Brønsted Acids: Chiral (Thio)urea Derivatives. In Comprehensive Enantioselective Organocatalysis, Ed.: Dalko, P. I., Wiley-VCH, Weinheim, 2013, pp. 315–341.
- 36 Zhao, Q.; Li, S.; Huang, K.; Wang, R.; Zhang, X. A Novel Chiral Bisphosphine-Thiourea Ligand for Asymmetric Hydrogenation of β,β-Disubstituted Nitroalkenes. Org. Lett. 2013, 15, 4014–4017.
- 37(a) Li, S.; Huang, K.; Cao, B.; Zhang, J.; Wu, W.; Zhang, X. Highly Enantioselective Hydrogenation of β,β-Disubstituted Nitro-alkenes. Angew. Chem. Int. Ed. 2012, 51, 8573−8576; (b) Yan, Q.; Liu, M.; Kong, D.; Zi, G.; Hou, G. Highly Efficient Iridium-Catalyzed Asymmetric Hydrogenation of β-Acylamino Nitroolefins. Chem. Commun. 2014, 50, 12870−12872; (c) Yu, Y.-B.; Cheng, L.; Li, Y.-P.; Fu, Y.; Zhu, S.-F.; Zhou, Q.-L. Enantioselective Iridium-Catalyzed Hydrogenation of β,β-Disubstituted Nitroalkenes. Chem. Commun. 2016, 52, 4812−4815.
- 38 Li, P.; Zhou, M.; Zhao, Q.; Wu, W.; Hu, X.; Dong, X.-Q.; Zhang, X. Synthesis of Chiral β-Amino Nitroalkanes via Rhodium-Catalyzed Asymmetric Hydrogenation. Org. Lett. 2016, 18, 40−43.
- 39 Zhang, T.; Jiang, J.; Yao, L.; Geng, H.; Zhang, X. Highly Efficient Synthesis of Chiral Aromatic Ketones via Rh-Catalyzed Asymmetric Hydrogenation of e β,β-Disubstituted Enones. Chem. Commun. 2017, 53, 9258−9261.
- 40 Wen, J.; Jiang, J.; Zhang, X. Rhodium-Catalyzed Asymmetric Hydrogenation of α,β-Unsaturated Carbonyl Compounds via Thiourea Hydrogen Bonding. Org. Lett. 2016, 18, 4451−4453.
- 41 Li, P.; Hu, X.; Dong, X.-Q.; Zhang, X. Rhodium/Bisphosphine-Thiourea-Catalyzed Enantioselective Hydrogenation of α,β-Unsaturated N-Acylpyrazoles. Chem. Commun. 2016, 52, 11677−11680.
- 42 Huang, Y.; Li, P.; Dong, X.-Q.; Zhang, X. Synthesis of chiral seven- membered β-substituted lactams via Rh-catalyzed asymmetric hydrogenation. Org. Biomol. Chem. 2018, 16, 8819–8823.
- 43 Han, Z.; Li, P.; Zhang, Z.; Chen, C.; Wang, Q.; Dong, X.-Q.; Zhang, X. Highly Enantioselective Synthesis of Chiral Succinimides via Rh/Bisphosphine-Thiourea-Catalyzed Asymmetric Hydrogenation. ACS Catal. 2016, 6, 6214−6218.
- 44 Han, Z.; Wang, R.; Gu, G.; Dong, X.-Q.; Zhang, X. Asymmetric Hydrogenation of Maleic Anhydrides Catalyzed by Rh/Bisphosphine-Thiourea: Efficient Construction of Chiral Succinic Anhydrides. Chem. Commun. 2017, 53, 4226−4229.
- 45 Zhang, Z.; Han, Z.; Gu, G.; Dong, X.-Q.; Zhang, X. Enantioselective Synthesis of Chiral 3-Substituted-3-silylpropionic Esters via Rhodium/ Bisphosphine-Thiourea-Catalyzed Asymmetric Hydrogenation. Adv. Synth. Catal. 2017, 359, 2585−2589.
- 46 Liu, G.; Li, A.; Qin, X.; Han, Z.; Dong, X.-Q.; Zhang, X. Efficient Access to Chiral β-Borylated Carboxylic Esters via Rh-Catalyzed Hydrogenation. Adv. Synth. Catal. 2019, 361, 2844−2848.
- 47 Liu, G.; Han, Z.; Dong, X. Q.; Zhang, X. Rh-Catalyzed Asymmetric Hydrogenation of β-Substituted-β-thio-α,β-unsaturated Esters: Expeditious Access to Chiral Organic Sulfides. Org. Lett. 2018, 20, 5636−5639.
- 48 Liu, G.; Zhang, H.; Huang, Y.; Han, Z.; Liu, G.; Liu, Y.; Dong, X.-Q.; Zhang, X. Efficient Synthesis of Chiral 2,3-Dihydro-benzo[b]-thiophene 1,1-Dioxides via Rh-Catalyzed Hydrogenation. Chem. Sci. 2019, 10, 2507−2512.
- 49 Sun, Y.; Jiang, J.; Guo, X.; Wen, J.; Zhang, X. Asymmetric Hydrogenation of α,β-Unsaturated Sulfones by a Rhodium/Thiourea-Bisphosphine Complex. Org. Chem. Front. 2019, 6, 1438−1441.
- 50 Yin, C.; Yang, T.; Pan, Y.; Wen, J.; Zhang, X. Rh-Catalyzed Asymmetric Hydrogenation of Unsaturated Medium-Ring NH Lactams: Highly Enantioselective Synthesis of N-Unprotected 2,3-Dihydro-1,5-benzothiazepinones. Org. Lett. 2020, 22, 920−923.
- 51 Yan, Q.; Kong, D.; Li, M.; Hou, G.; Zi, G. Highly Efficient Rh-Catalyzed Asymmetric Hydrogenation of α,β-Unsaturated Nitriles. J. Am. Chem. Soc. 2015, 137, 10177−10181.
- 52 Li, X.; You, C.; Yang, Y.; Yang, Y.; Li, P.; Gu, G.; Chung, L. W.; Lv, H.; Zhang, X. Rhodium-Catalyzed Asymmetric Hydrogenation of β-Cyanocinnamic Esters with the Assistance of a Single Hydrogen Bond in a Precise Position. Chem. Sci. 2018, 9, 1919−1924.
- 53 Yin, X.; Huang, Y.; Chen, Z.; Hu, Y.; Tao, L.; Zhao, Q.; Dong, X.-Q.; Zhang, X. Enantioselective Access to Chiral 2-Substituted 2,3-Dihydrobenzo[1,4]dioxane Derivatives through Rh-Catalyzed Asymmetric Hydrogenation. Org. Lett. 2018, 20, 4173−4177.
- 54 Han, Z.; Guan, Y.-Q.; Liu, G.; Wang, R.; Yin, X.; Zhao, Q.; Cong, H.; Dong, X.-Q.; Zhang, X. Iridium-Catalyzed Asymmetric Hydrogenation of Tetrasubstituted α-Fluoro-β-enamino Esters: Efficient Access to Chiral α-Fluoro-β-amino Esters with Two Adjacent Tertiary Stereocenters. Org. Lett. 2018, 20, 6349−6353.
- 55 Zhao, Q.; Wen, J.; Tan, R.; Huang, K.; Metola, P.; Wang, R.; Anslyn, E. V.; Zhang, X. Rhodium-Catalyzed Asymmetric Hydrogenation of Unprotected NH Imines Assisted by a Thiourea. Angew. Chem. Int. Ed. 2014, 53, 8467−8470.
- 56 Li, P.; Huang, Y.; Hu, X.; Dong, X.-Q.; Zhang, X. Access to Chiral Seven- Member Cyclic Amines via Rh-Catalyzed Asymmetric Hydrogenation. Org. Lett. 2017, 19, 3855−3858.
- 57 Wen, J.; Tan, R.; Liu, S.; Zhao, Q.; Zhang, X. Strong Bronsted Acid Promoted Asymmetric Hydrogenation of Isoquinolines and Quinolines Catalyzed by a Rh-Thiourea Chiral Phosphine Complex via Anion Binding. Chem. Sci. 2016, 7, 3047−3051.
- 58 Wen, J.; Fan, X.; Tan, R.; Chien, H.-C.; Zhou, Q.; Chung, L. W.; Zhang, X. Brønsted-Acid-Promoted Rh-Catalyzed Asymmetric Hydrogenation of N-Unprotected Indoles: A Cocatalysis of Transition Metal and Anion Binding. Org. Lett. 2018, 20, 2143−2147.
- 59 Han, Z.; Liu, G.; Wang, R.; Dong, X.-Q.; Zhang, X. Highly Efficient Ir-Catalyzed Asymmetric Hydrogenation of Benzoxazinones and Derivatives with a Brønsted Acid Cocatalyst. Chem. Sci. 2019, 10, 4328−4333.
- 60 Yang, T.; Yin, Q.; Gu, G.; Zhang, X. A One-Pot Process for the Enantioselective Synthesis of Tetrahydroquinolines and Tetrahydroisoquinolines via Asymmetric Reductive Amination (ARA). Chem. Commun. 2018, 54, 7247−7250.
- 61 Yang, T.; Sun, Y.; Wang, H.; Lin, Z.; Wen, J.; Zhang, X. Iridium-Catalyzed Enantioselective Hydrogenation of Oxocarbenium Ions: A Case of Ionic Hydrogenation. Angew. Chem. Int. Ed. 2020, 59, 6108−6114.
- 62(a) Phipps, R. J.; Hamilton, G. L.; Toste, F. D. The progression of chiral anions from concepts to applications in asymmetric catalysis. Nat. Chem. 2012, 4, 603–614; (b) Brak, K.; Jacobsen, E. N. Asymmetric Ion-Pairing Catalysis. Angew. Chem. Int. Ed. 2013, 52, 534–561; (c) Visco, M. D.; Attard, J.; Guan, Y.; Mattson, A. E. Anion-Binding Catalyst Designs for Enantioselective Synthesis. Tetrahedron Lett. 2017, 58, 2623–2628.
- 63(a) Chen, C.; Wang, H.; Zhang, Z.; Jin, S.; Wen, S.; Ji, J.; Chung, L. W.; Dong, X.-Q.; Zhang, X. Ferrocenyl Chiral Bisphosphorus Ligands for Highly Enantioselective Asymmetric Hydrogenation via Noncovalent Ion Pair Interaction. Chem. Sci. 2016, 7, 6669−6673; (b) Chen, C.; Wen, S.; Dong, X.-Q.; Zhang, X. Highly Stereoselective Synthesis and Application of P-Chiral Ferrocenyl Bisphosphorus Ligands for Asymmetric Hydrogenation. Org. Chem. Front. 2017, 4, 2034−2038.
- 64 Wen, S.; Chen, C.; Du, S.; Zhang, Z.; Huang, Y.; Han, Z.; Dong, X.-Q.; Zhang, X. Highly Enantioselective Asymmetric Hydrogenation of Carboxy-Directed α,α-Disubstituted Terminal Olefins via the Ion Pair Noncovalent Interaction. Org. Lett. 2017, 19, 6474−6477.
- 65 Yin, X.; Chen, C.; Dong, X.-Q.; Zhang, X. Rh/Wudaphos-Catalyzed Asymmetric Hydrogenation of Sodium α-Arylethenylsulfonates: A Method to Access Chiral α-Arylethylsulfonic Acids. Org. Lett. 2017, 19, 2678−2681.
- 66 Chen, C.; Wen, S.; Geng, M.; Jin, S.; Zhang, Z.; Dong, X.-Q.; Zhang, X. A New Ferrocenyl Bisphosphorus Ligand for the Asymmetric Hydrogenation of α-Methylene-γ-Keto-Carboxylic Acids. Chem. Commun. 2017, 53, 9785−9788.
- 67 Yin, X.; Chen, C.; Li, X.; Dong, X.-Q.; Zhang, X. Rh/SPO-WudaPhos- Catalyzed Asymmetric Hydrogenation of α-Substituted Ethenylphosphonic Acids via Noncovalent Ion-Pair Interaction. Org. Lett. 2017, 19, 4375−4378.
- 68 Nishiyama, H.; Sakaguchi, H.; Nakamura, T.; Horihata, M.; Kondo, M.; Itoh, K. Chiral and C2-Symmetrical Bis(oxazolinylpyridine)-rhodium(III) Complexes: Effective Catalysts for Asymmetric Hydrosilylation of Ketones. Organometallics 1989, 8, 846–848.
- 69(a) Jiang, Y.; Plew, D.; Murtuza, S.; Zhang, X. Synthesis of (1R,1R’)-2,6-Bis[1-(diphenylphosphino)ethyl]pyridine and its Application in Asymmetric Transfer Hydrogenation. Tetrahedron Lett. 1996, 37, 797–800; (b) Sablong, R.; Osborn, J. The Asymmetric Hydrogenation of Imines using Tridentate C2 Diphosphine Complexes of Iridium(I) and Rhodium(I). Tetrahedron Lett. 1996, 37, 4937–4940; (c) Jiang, Y.; Jiang, Q.; Zhu, G.; Zhang, X. Highly Effective NPN-type Tridentate Ligands for Asymmetric Transfer Hydrogenation of Ketones. Tetrahedron Lett. 1997, 38, 215–218; (d) Jiang, Y.; Jiang, Q.; Zhang, X. A New Chiral Bis(oxazolinylmethyl)amine Ligand for Ru-Catalyzed Asymmetric Transfer Hydrogenation of Ketones. J. Am. Chem. Soc. 1998, 120, 3817–3818.
- 70 Li, W.; Hou, G.; Wang, C.; Jiang, Y.; Zhang, X. Asymmetric Hydrogenation of Ketones Catalyzed by a Ruthenium(ii)-Indan–Ambox complex. Chem. Commun. 2010, 46, 3979–3981.
- 71 Hayashi, T.; Mise, T.; Fukushima, M.; Kagotani, M.; Nagashima, N.; Hamada, Y.; Matsumoto, A.; Kawakami, S.; Konishi, M.; Yamamoto, K.; Kumada, M. B. Asymmetric Synthesis Catalyzed by Chiral Ferrocenylphosphine–Transition Metal Complexes. I. Preparation of Chiral Ferrocenylphosphines. Chem. Soc. Jpn. 1980, 53, 1138–1151.
- 72 Gleiter, R.; Bleiholder, C.; Rominger, F. α-Metallocenylmethylium Ions and Isoelectronic Fulvene Complexes of d6 to d9 Metals. Structural Considerations. Organometallics 2007, 26, 4850–4859.
- 73 Wu, W.; Liu, S.; Duan, M.; Tan, X.; Chen, C.; Xie, Y.; Lan, Y.; Dong, X.; Zhang, X. Iridium Catalysts with f-Amphox Ligands: Asymmetric Hydrogenation of Simple Ketones. Org. Lett. 2016, 18, 2938–2941.
- 74 Yu, J.; Long, J.; Yang, Y.; Wu, W.; Xue, P.; Chung, L.; Dong, X.; Zhang, X. Iridium-Catalyzed Asymmetric Hydrogenation of Ketones with Accessible and Modular Ferrocene-Based Amino-phosphine Acid (f-Ampha) Ligands. Org. Lett. 2017, 19, 690–693.
- 75 Liang, Z.; Yang, T.; Gu, G.; Dang, L.; Zhang, X. Scope and Mechanism on Iridium-f-Amphamide Catalyzed Asymmetric Hydrogenation of Ketones. Chin. J. Chem. 2018, 36, 851–856.
- 76 Yin, C.; Wu, W.; Hu, Y.; Tan, X.; You, C.; Liu, Y.; Chen, Z.; Dong, X.; Zhang, X. Iridium-Catalyzed Asymmetric Hydrogenation of HalogenatedKetones for the Efficient Construction of Chiral Halohydrins. Adv. Synth. Catal. 2018, 360, 2119–2124.
- 77 Zhang, X.; Gu, G. Method for Synthesizing Chiral Alpha-Hydroxy Amide by Catalyzing Prochiral Alpha-Keto-Amide. CN 107417562 A, 2017.
- 78 Ding, X.; Wang, S. Asymmetric Hydrogenation Method of Alpha- Ketone Amide Compound. CN 108546238 A, 2018.
- 79 Wang, S.; Yu, Y.; Wen, J.; Zhang, X. Iridium/f-Amphox-Catalyzed Asymmetric Hydrogenation of Styrylglyoxylamides. Synlett 2018, 29, 2203–2207.
- 80 Hu, Y.; Wu, W.; Dong, X.; Zhang, X. Efficient Access to Chiral 1,2-Amino Alcohols via Ir/f-Amphox-Catalyzed Asymmetric Hydrogenation of α-Amino Ketones. Org. Chem. Front. 2017, 4, 1499–1502.
- 81 Yuan, M.; Xie, J.; Yang, X.; Zhou, Q. Enantioselective Synthesis of Chiral 1,2-Amino Alcohols via Asymmetric Hydrogenation of α-Amino Ketones with Chiral Spiro Iridium Catalysts. Synthesis 2014, 46, 2910–2916.
- 82 Zhang, X.; Hu, Y.; Wu, W.; Dong, X. Method for Efficiently Synthesizing Chiral 1,2-Amino Alcohol by Catalyzing Alpha-Aminoketone through Ir/f-amphox. CN 107021884 A, 2017.
- 83 Wu, W.; You, C.; Yin, C.; Liu, Y.; Dong, X.; Zhang, X. Enantioselective and Diastereoselective Construction of Chiral Amino Alcohols by Iridium–f-Amphox-Catalyzed Asymmetric Hydrogenation via Dynamic Kinetic Resolution. Org. Lett. 2017, 19, 2548–2551.
- 84 Yu, J.; Duan, M.; Wu, W.; Qi, X.; Xue, P.; Lan, Y.; Dong, X.; Zhang, X. Readily Accessible and Highly Efficient Ferrocene-Based Amino- Phosphine-Alcohol (f-Amphol) Ligands for Iridium-Catalyzed Asymmetric Hydrogenation of Simple Ketones. Chem.-Eur. J. 2017, 23, 970–975.
- 85 Zhang, X. Chiral Tridentate Nitrogen-Phosphine-Oxygen Ligands and Application of Related Ligands in Asymmetric Catalytic Reactions. CN 105732725 A, 2016.
- 86 Gu, G.; Lu, J.; Yu, O.; Wen, J.; Yin, Q.; Zhang, X. Enantioselective and Diastereoselective Ir-Catalyzed Hydrogenation of α-Substituted β-Ketoesters via Dynamic Kinetic Resolution. Org. Lett. 2018, 20, 1888–1892.
- 87 Zhang, X.; Yu, J. Chiral Tridentate Phosphonic Amine Ligand and Application thereof in Asymmetric Catalytic Reaction. CN 106632511 A, 2017.
- 88 Gong, Q.; Wen, J.; Zhang, X. Desymmetrization of Cyclic 1,3-Diketones via Ir-Catalyzed Hydrogenation: An Efficient Approach to Cyclic Hydroxy Ketones with a Chiral Quaternary Carbon. Chem. Sci. 2019, 10, 6350–6353.
- 89 Gu, G.; Yang, T.; Lu, J.; Wen, J.; Dang, L.; Zhang, X. Iridium/f-Ampha- Catalyzed Asymmetric Hydrogenation of Aromatic α-Keto esters. Org. Chem. Front. 2018, 5, 1209–1212.
- 90 Tao, L.; Yin, C.; Dong, X.; Zhang, X. Efficient Synthesis of Chiral β-Hydroxy Sulfones via Iridium-Catalyzed Hydrogenation. Org. Biomol. Chem. 2019, 17, 785–788.




