Frustrated Lewis Pair Catalysis: It Takes Two to Make a Thing Go Right
Xinyue Tan
Department of Chemistry and Shanghai Key Laboratory of Molecular Catalysis and Innovative Materials, Fudan University, Songhu Road 2005, Shanghai, 200438 China
Search for more papers by this authorCorresponding Author
Huadong Wang
Department of Chemistry and Shanghai Key Laboratory of Molecular Catalysis and Innovative Materials, Fudan University, Songhu Road 2005, Shanghai, 200438 China
E-mail: [email protected]Search for more papers by this authorXinyue Tan
Department of Chemistry and Shanghai Key Laboratory of Molecular Catalysis and Innovative Materials, Fudan University, Songhu Road 2005, Shanghai, 200438 China
Search for more papers by this authorCorresponding Author
Huadong Wang
Department of Chemistry and Shanghai Key Laboratory of Molecular Catalysis and Innovative Materials, Fudan University, Songhu Road 2005, Shanghai, 200438 China
E-mail: [email protected]Search for more papers by this authorAbstract
Since the introduction of the concept of frustrated Lewis pair by Stephan in 2006, the frustrated Lewis pair (FLP) chemistry has evolved into a rich and fruitful research area which is largely responsible for the renaissance of main group chemistry in recent years. Among many applications of FLP, design of catalytic systems based on the concept of FLP, pioneered by Stephan, Chen and others, provides a powerful arsenal that has been applied to organic synthesis and polymerization. This article will highlight key advances in the development of FLP related catalysis.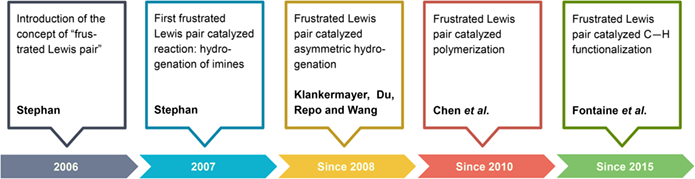
References
- 1 Xu, M.; Qu, Z.-W.; Grimme, S.; Stephan, D. W. J. Am. Chem. Soc. 2021, 143, 2, 634–638.
- 2 Jupp, R.; Stephan, D. W. Trends Chem. 2019, 1, 35–48.
- 3 Xu, M.; Jupp, A. R.; Qu, Z.-W.; Grimme, S.; Stephan, D. W. Angew. Chem. Int. Ed. 2018, 57, 11050–11054.
- 4 Fan, L.; Jupp, A. R.; Stephan, D. W. J. Am. Chem. Soc. 2018, 140, 8119–8123.
- 5 Tang, C.; Liang, Q.; Jupp, A. R.; Johnstone, T. C.; Neu, R. C.; Song, D.; Grimme, S.; Stephan, D. W. Angew. Chem. Int. Ed. 2017, 56, 16588–16592
- 6 Liu, L.; Cao, L. L.; Shao, T.; Menard, G.; Stephan, D. W. Chem 2017, 3, 259–267.
- 7 Stephan, D. W. Science 2016, 354, aaf7229.
- 8 Stephan, D. W. J. Am. Chem. Soc. 2015, 137, 10018–10032.
- 9 Stephan, D. W. Acc. Chem. Res. 2015, 48, 306–316.
- 10 Stephan, D. W.; Erker, G. Angew. Chem. Int. Ed. 2015, 54, 6400–6441.
- 11 Stephan, D. W.; Erker, G. Angew. Chem. Int. Ed. 2010, 49, 46–76.
- 12 Chase, P.; Jurca, T.; Stephan, D. W. Angew. Chem. Int. Ed. 2007, 46, 8050–8053.
- 13 Welch, G. C.; Stephan, D. W. J. Am. Chem. Soc. 2007, 129, 1880–1881.
- 14 Welch, G. C.; San Juan, R.; Masuda, J. D.; Stephan, D. W. Science 2006, 314, 1124–1126.
- 15 Lewis, G. N. Valence and the Structure of Atoms and Molecules, The Chemical Catalogue Company, New York, 1923.
- 16 Hisashi, Y. Lewis Acids in Organic Synthesis, WILEY-VCH Verlag GmbH, 2000.
- 17 Denmark, S. E.; Beutner, G. L. Lewis Base Catalysis in Organic Synthesis. Angew. Chem. Int. Ed. 2008, 47, 1560–1638.
- 18 Stephan, D. W.; Erker, G. Frustrated Lewis Pairs: Metal-free Hydrogen Activation and More. Angew. Chem. Int. Ed. 2010, 49, 46–76.
- 19 Stephan, D. W.; Erker, G. Frustrated Lewis Pair Chemistry: Development and Perspectives. Angew. Chem. Int. Ed. 2015, 54, 6400–6441.
- 20 Stephan, D. W. Frustrated Lewis Pairs: From Concept to Catalysis. Acc. Chem. Res. 2015, 48, 306–316.
- 21 Stephan, D. W. Frustrated Lewis Pairs. J. Am. Chem. Soc. 2015, 137, 10018–10032.
- 22Stephan, D. W. The Broadening Reach of Frustrated Lewis Pair Chemistry. Science 2016, 354, 1248.
- 23 Lam, J.; Szkop, K. M.; Mosaferi, E.; Stephan, D. W. FLP Catalysis: Main Group Hydrogenations of Organic Unsaturated Substrates. Chem. Soc. Rev. 2019, 48, 3592–3612.
- 24 Welch, G. C.; San Juan, R. R.; Masuda, J. D.; Stephan, D. W. Reversible, Metal-Free Hydrogen Activation. Science 2006, 314, 1124–1126
- 25 Welch, G. C.; Stephan, D. W. Facile Heterolytic Cleavage of Dihydrogen by Phosphines and Boranes. J. Am. Chem. Soc. 2007, 129, 1880–1881.
- 26 Rokob, T. A.; Hamza, A.; Stirling, A.; Soós, T.; Pápai, I. Turning Frustration into Bond Activation: A Theoretical Mechanistic Study on Heterolytic Hydrogen Splitting by Frustrated Lewis Pairs. Angew. Chem. Int. Ed. 2008, 47, 2435–2438.
- 27 Grimme, S.; Kruse, H.; Goerigk, L.; Erker, G. The Mechanism of Dihydrogen Activation by Frustrated Lewis Pairs Revisited. Angew. Chem. Int. Ed. 2010, 49, 1402–1405.
- 28 Brown, L. C.; Hogg, J. M.; Gilmore, M.; Moura, L.; Imberti, S.; Gärtner, S.; Gunaratne, H. Q. N.; O'Donnell, R. J.; Artioli, N.; Holbrey, J. D.; Swadźba-Kwaśny, M. Frustrated Lewis Pairs in Ionic Liquids and Molecular Solvents – a Neutron Scattering and NMR Study of Encounter Complexes. Chem. Commun. 2018, 54, 8689–8692.
- 29 Hounjet, L. J.; Bannwarth, C.; Garon, C. N.; Caputo, C. B.; Grimme, S.; Stephan, D. W. Combinations of Ethers and B(C6F5)3 Function as Hydrogenation Catalysts. Angew. Chem. Int. Ed. 2013, 52, 7492–7495.
- 30 Lam, J.; Szkop, K. M.; Mosaferi, E.; Stephan, D. W. FLP catalysis: main group hydrogenations of organic unsaturated substrates. Chem. Soc. Rev. 2019, 48, 3592–3612.
- 31 Chase, P. A.; Welch, G. C.; Jurca, T.; Stephan, D. W. Metal-Free Catalytic Hydrogenation. Angew. Chem. Int. Ed. 2007, 46, 8050–8053.
- 32 Spies, P.; Schwendemann, S.; Lange, S.; Kehr, G.; Fröhlich, R.; Erker, G. Metal-Free Catalytic Hydrogenation of Enamines, Imines, and Conjugated Phosphinoalkenylboranes. Angew. Chem. Int. Ed. 2008, 47, 7543–7546.
- 33 Wang, H.; Kehr, G.; Fröhlich, R.; Erker, G. Heterolytic dihydrogen activation with the 1,8-bis(diphenylphosphino)naphthalene/B(C6F5)3 pair and its application for metal-free catalytic hydrogenation of silyl enol ethers Chem. Commun. 2008, 5966–5968.
- 34 Scott, D. J.; Fuchter, M. J.; Ashley, A. E. Nonmetal Catalyzed Hydrogenation of Carbonyl Compounds. J. Am. Chem. Soc. 2014, 136, 15813–15816.
- 35 Mahdi, T.; Stephan, D. W. Enabling Catalytic Ketone Hydrogenation by Frustrated Lewis Pairs. J. Am. Chem. Soc. 2014, 136, 15809–15812.
- 36 Greb, L.; Oña-Burgos, P.; Schirmer, B.; Grimme, S.; Stephan, D. W.; Paradies, J. Metal-free Catalytic Olefin Hydrogenation: Low-Temperature H2 Activation by Frustrated Lewis Pairs. Angew. Chem. Int. Ed. 2012, 51, 10164–10168.
- 37 Nagarkar, A. A.; Kilbinger, A. F. M. Catalytic Living Ring-opening Metathesis Polymerization. Nat. Chem. 2015, 7, 718–723.
- 38 Meng, W.; Feng, X.; Du, H. Asymmetric Catalysis with Chiral Frustrated Lewis Pairs. Chin. J. Chem. 2020, 38, 625–634.
- 39 Chen, D.; Klankermayer, J. Metal-free Catalytic Hydrogenation of Imines with Tris(perfluorophenyl)borane. Chem. Commun. 2008, 2130–2131.
- 40 Chen, D.; Wang, Y.; Klankermayer, J. Enantioselective Hydrogenation with Chiral Frustrated Lewis Pairs. Angew. Chem. Int. Ed. 2010, 49, 9475–9478.
- 41 Meng, W.; Feng, X. Q.; Du, H. F. Frustrated Lewis Pairs Catalyzed Asymmetric Metal-Free Hydrogenations and Hydrosilylations. Acc. Chem. Res. 2018, 51, 191–201.
- 42 Liu, X.; Wang, Q.; Han, C.; Feng, X.; Du, H. Chiral Frustrated Lewis Pairs Catalyzed Highly Enantioselective Hydrosilylations of Ketones. Chin. J. Chem. 2019, 37, 663–666.
- 43 Meng, W.; Feng, X.; Du, H. Asymmetric Catalysis with Chiral Frustrated Lewis Pairs. Chin. J. Chem. 2020, 38, 625–634.
- 44 Liu, Y. B.; Du, H. F. Chiral Dienes as “Ligands” for Borane-catalyzed Metal-free Asymmetric Hydrogenation of Imines. J. Am. Chem. Soc. 2013, 135, 6810–6813.
- 45 Tu, X. S.; Zeng, N. N.; Li, R. Y.; Zhao, Y. Q.; Xie, D. Z.; Peng, Q.; Wang, X. C. C2-symmetric Bicyclic Bisborane Catalysts: Kinetic or Thermo- dynamic Products of a Reversible Hydroboration of Dienes. Angew. Chem. Int. Ed. 2018, 57, 15096–15100.
- 46 Lindqvist, M.; Borre, K.; Axenov, K.; Kótai, B.; Nieger, M.; Leskelä, M.; Pápai, I.; Repo, T., Chiral Molecular Tweezers: Synthesis and Reactivity in Asymmetric Hydrogenation. Chiral Molecular Tweezers: Synthesis and Reactivity in Asymmetric Hydrogenation. J. Am. Chem. Soc. 2015, 137, 4038–4041.
- 47 Stephan, D. W. ; Greenberg, S.; Graham, T. W.; Chase, P.; Hastie, J. J.; Geier, S. J.; Farrell, J. M.; Brown, C. C.; Heiden, Z. M.; Welch, G. C.; Ullrich, M. Metal-Free Catalytic Hydrogenation of Polar Substrates by Frustrated Lewis Pairs. Inorg. Chem. 2011, 50, 12338–12348
- 48 Gao, B.; Feng, X.; Meng, W.; Du, H. Asymmetric Hydrogenation of Ketones and Enones with Chiral Lewis Base Derived Frustrated Lewis Pairs. Angew. Chem. Int. Ed. 2020, 59, 4498–4504.
- 49 Bavykina, A.; Kolobov, N.; Khan, I. S.; Bau, J. A.; Ramirez, A.; Gascon, J. Metal–Organic Frameworks in Heterogeneous Catalysis: Recent Progress, New Trends, and Future Perspectives. Chem. Rev. 2020, 120, 8468–8535.
- 50 Niu, Z.; Gunatilleke, W. D. C. B.; Sun, Q.; Lan, P. C.; Perman, J.; Ma, J.-G.; Cheng, Y.; Aguila, B.; Ma, S. Metal-organic framework anchored with Lewis pair as a new paradigm for catalysis. Chem 2018, 4, 2587–2599.
- 51 Niu, Z.; Zhang, W.; Lan, P. C.; Aguila, B.; Ma, S. Promoting Frustrated Lewis Pairs for Heterogeneous Chemoselective Hydrogenation via the Tailored Pore Environment within Metal–Organic Frameworks. Angew. Chem. Int. Ed. 2019, 58, 7420–7424.
- 52 Ding, Y.; Huang, X.; Yi, X.; Qiao, Y.; Sun, X.; Zheng, A.; Su, D. S. A Heterogeneous Metal-Free Catalyst for Hydrogenation: Lewis Acid–Base Pairs Integrated into a Carbon Lattice. Angew. Chem. Int. Ed. 2018, 57, 13800–13804.
- 53 Scott, D. J.; Fuchter, M. J.; Ashley, A. E. Activation of H2 by Phosphinoboranes R2PB(C6F5)2. J. Am. Chem. Soc. 2008, 130, 12632–12633.
- 54 Ménard, G.; Stephan, D. W. C-H Activation of Isobutylene Using Frustrated Lewis Pairs: Aluminum and Boron σ-Allyl Complexes. Angew. Chem. Int. Ed. 2012, 51, 4409–4412.
- 55 Dureen, M. A.; Stephan, D. W. Terminal Alkyne Activation by Frustrated and Classical Lewis Acid/Phosphine Pairs. J. Am. Chem. Soc. 2009, 131, 8396–8397
- 56 Jiang, C.; Blacque, O.; Berke, H. Activation of Terminal Alkynes by Frustrated Lewis Pairs. Organometallics 2010, 29, 125–133.
- 57 Légaré, M. A.; Courtemanche, M. A.; Rochette, E.; Fontaine, F. G. Metal-free Catalytic C-H Bond Activation and Borylation of Heteroarenes. Science 2015, 349, 513–516.
- 58 Shang, M.; Cao, M.; Wang, Q.; Wasa, M. Enantioselective Direct Mannich-Type Reactions Catalyzed by Frustrated Lewis Acid/ Brønsted Base Complexes. Angew. Chem. Int. Ed. 2017, 56, 13338–13341.
- 59 Hong, M.; Chen, J. W.; Chen, E. Y. X. Polymerization of Polar Monomers Mediated by Main-Group Lewis Acid–Base Pairs. Chem. Rev. 2018, 118, 10551–10616.
- 60 McGraw, M. L.; Chen, E. Y. X. Lewis Pair Polymerization: Perspective on a Ten-Year Journey. Macromolecules 2020, 53, 6102–6122.
- 61 Zhang, Y.; Miyake, G. M.; Chen, E. Y. X. Alane-Based Classical and Frustrated Lewis Pairs in Polymer Synthesis: Rapid Polymerization of MMA and Naturally Renewable Methylene Butyrolactones into High-Molecular-Weight Polymers. Angew. Chem. Int. Ed. 2010, 49, 10158–10162.
- 62 Bai, Y.; He, J.; Zhang, Y. Ultra-High-Molecular-Weight Polymers Produced by the Immortal Phosphine-Based Catalyst System. Angew. Chem. Int. Ed. 2018, 57, 17230–17234.
- 63 McGraw, M. L.; Clarke, R. W.; Chen, E. Y. X. Compounded Sequence Control in Polymerization of One-Pot Mixtures of Highly Reactive Acrylates by Differentiating Lewis Pairs. J. Am. Chem. Soc. 2020, 142, 5969–5973.
- 64 Wang, X. J.; Hong, M. Lewis-Pair-Mediated Selective Dimerization and Polymerization of Lignocellulose-Based β-Angelica Lactone into Biofuel and Acrylic Bioplastic. Angew. Chem. Int. Ed. 2020, 59, 2664–2668.
- 65 Ghosh, D.; Sinhababu, S.; Santarsiero, B. D.; Mankad, N. P. A W/Cu Synthetic Model for the Mo/Cu Cofactor of Aerobic CODH Indicates That Biochemical CO Oxidation Requires a Frustrated Lewis Acid/ Base Pair. J. Am. Chem. Soc. 2020, 142, 12635–12642.
- 66 Liu, L.; Cao, L. L.; Shao, Y.; Ménard, G.; Stephan, D. W. A Radical Mechanism for Frustrated Lewis Pair Reactivity. Chem 2017, 3, 259–267.
- 67 Holtrop, F.; Jupp, A. R.; Kooij, B. J.; van Leest, N. P.; de Bruin, B.; Slootweg, J. C. Single-electron Transfer in Frustrated Lewis Pair Chemistry. Angew. Chem. Int. Ed. 2020, 59, 22210–22216.
- 68 Dasgupta, A.; Richards, E.; Melen, R. L. Frustrated Radical Pairs: Insights from EPR Spectroscopy. Angew. Chem. Int. Ed. 2021, 60, 53–65.
- 69 Aramaki, Y.; Imaizumi, N.; Hotta, M.; Kumagai, J.; Ooi, T. Exploiting single-electron transfer in Lewis pairs for catalytic bond-forming reactions. Chem. Sci. 2020, 11, 4305–4311




