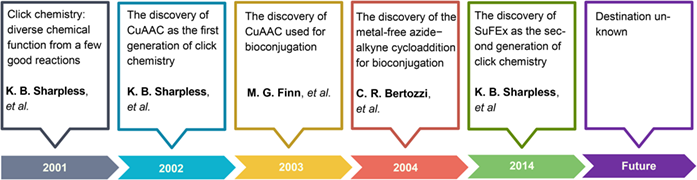
Click Chemistry: Evolving on the Fringe
Long Xu
Key Laboratory of Organofluorine Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Ling-Ling Road, Shanghai, 200032 China
Search for more papers by this authorCorresponding Author
Jiajia Dong
Key Laboratory of Organofluorine Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Ling-Ling Road, Shanghai, 200032 China
E-mail: [email protected]Search for more papers by this authorLong Xu
Key Laboratory of Organofluorine Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Ling-Ling Road, Shanghai, 200032 China
Search for more papers by this authorCorresponding Author
Jiajia Dong
Key Laboratory of Organofluorine Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Ling-Ling Road, Shanghai, 200032 China
E-mail: [email protected]Search for more papers by this authorSummary
The article herein briefly introduces the story of the birth of click chemistry and its evolution after that. A new angle to interpret click reactions was proposed using the “reactivity-availability-functionality” trilogy. CuAAC (Copper-catalyzed azide-alkyne cycloaddition), the most popular click reaction by far, was revisited along with the thiol-ene, metal-free AAC, SuFEx (Sulfur(VI) fluoride exchange) and the lately discovered diazotransfer process. By encountering more and more near-perfect reactions, click chemistry is evolving and expanding on the fringe of the chemistry and different scientific disciplines, destination unknown.
References
- 1Sharpless, K. B. Priestley Medal address 2019: A simple life-finding function and making connections. Chem. Eng. News 2019, 97, 30–35.
- 2
Kolb, H. C.; Finn, M. G.; Sharpless, K. B. Click chemistry: diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021.
10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5 CAS PubMed Web of Science® Google Scholar
- 3(a) Rostovtsev, V. V.; Green, L. G.; Fokin, V. V.; Sharpless, K. B. A Stepwise Huisgen cycloaddition process: copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599;
10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4 CAS PubMed Web of Science® Google Scholar(b) Tornøe, C. W.; Christensen, C.; Melda, M. Peptidotriazoles on solid phase: [1,2,3]-triazoles by regiospecific copper(I)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J. Org. Chem. 2002, 67, 3057–3064.
- 4(a) Huisgen, R. 1,3-Dipolar cycloadditions. Past and future. Angew. Chem. Int. Ed. 1963, 2, 565–632;
10.1002/anie.196305651 Google Scholar(b) Huisgen, R. Kinetics and mechanism of 1,3-dipolar cycloadditions. Angew. Chem. Int. Ed. 1963, 2, 633–696.10.1002/anie.196306331 Google Scholar
- 5 Huisgen, R. Kinetics and Reaction Mechanisms: Selected Examples from the Experience of Forty Years. Pure Appl. Chem. 1989, 61, 613–628.
- 6 Huisgen, R. In 1,3-Dipolar Cycloaddition Chemistry, Vol. 1, Ed.: Padwa, A., Wiley, New York, 1984, pp. 1–176.
- 7 Himo, F.; Lovell, T.; Hilgraf, R.; Rostovtsev, V. V.; Noodleman, L.; Sharpless, K. B.; Fokin, V. V. Copper(I)-catalyzed synthesis of azoles. DFT study predicts unprecedented reactivity and intermediates. J. Am. Chem. Soc. 2005, 127, 210–216.
- 8 Salic, A.; Mitchison, T. J. A chemical method for fast and sensitive detection of DNA synthesis in vivo. Proc. Natl. Acad. Sci. U. S. A. 2008, 105, 2415–2420.
- 9 Bertrand, D.; Bertrand, S.; Neveu, E.; Fernandes, P. Molecular characterization of off-target activities of telithromycin: a potential role for nicotinic acetylcholine receptors. Antimicrob. Agents Chemother. 2010, 54, 5399–5402.
- 10 Fernandes, P.; Martens, E.; Bertrand, D.; Pereira, D. The solithromycin journey—it is all in the chemistry. Bioorg. Med. Chem. 2016, 24, 6420–6428.
- 11 Sumerlin, B. S.; Tsarevsky, N. V.; Louche, G.; Lee, R. Y.; Matyjaszewski, K. Highly efficient “click” functionalization of poly(3-azidopropyl methacrylate) prepared by ATRP. Macromolecules 2005, 38, 7540–7545.
- 12 Pressly, E. D.; Hawker, C. J. Methods for fixing hair and skin. US 9095518B2, 2015.
- 13 Agard, N. J.; Prescher, J. A.; Bertozzi, C. R. A Strain-Promoted [3 + 2] Azide−Alkyne Cycloaddition for Covalent Modification of Biomolecules in Living Systems. J. Am. Chem. Soc. 2004, 126, 15046–15047.
- 14 Dong, J.; Krasnova, L.; Finn, M. G.; Sharpless, K. B. Sulfur(VI) fluoride exchange (SuFEx): another good reaction for click chemistry. Angew. Chem. Int. Ed. 2014, 53, 9430–9448.
- 15 Barrow, A. S.; Smedley, C. J.; Zheng, Q.; Li, S.; Dong, J.; Moses, J. E. The growing applications of SuFEx click chemistry. Chem. Soc. Rev. 2019, 48, 4731–4758.
- 16 Dong, J.; Sharpless, K. B.; Kwisnek, L.; Oakdale, J. S.; Fokin, V. V. SuFEx-Based Synthesis of Polysulfates. Angew. Chem. Int. Ed. 2014, 53, 9466–9470.
- 17(a) Corbett, T. H.; Leopold, W. R.; Dykes, D. J.; Roberts, B. J.; Griswold, D. P.; Schabel, F. M. Toxicity and anticancer activity of a new triazine antifolate (NSC 127755). Cancer Res. 1982, 42, 1707–1715; (b) Baker, B. R. Irreversible enzyme inhibitors. CXLIX. tissue-specific irreversible inhibitors of dihydrofolic reductase. Acc. Chem. Res. 1969, 2, 129–136; (c) Baker, B. B.; Wood, W. F. Irreversible enzyme inhibitors. CXLVIII. active-site-directed irreversible inhibitors of guanine deaminase derived from 9-phenylguanine bearing a terminal sulfonyl fluoride. J. Med. Chem. 1969, 12, 216–220; (d) Chen, W.; Dong, J.; Plate, L.; Mortenson, D. E.; Brighty, G. J.; Li, S.; Liu, Y.; Galmozzi, A.; Lee, P. S.; Hulce, J. J.; Cravatt, B. F.; Saez, E.; Powers, E. T.; Wilson, I. A.; Sharpless, K. B.; Kelly, J. W. Arylfluorosulfates inactivate intracellular lipid binding protein(s) through chemoselective SuFEx reaction with a binding site tyr residue. J. Am. Chem. Soc. 2016, 138, 7353–7364; (e) Mortenson, D. E.; Brighty, G. J.; Plate, L.; Bare, G.; Chen, W.; Li, S.; Wang, H.; Cravatt, B. F.; Forli, S.; Powers, E. T.; Sharpless, K. B.; Wilson, I. A.; Kelly, J. W. Inverse drug discovery” strategy to identify proteins that are targeted by latent electrophiles as exemplified by aryl fluorosulfates. J. Am. Chem. Soc. 2018, 140, 200–210; (f) Narayanan, A.; Jones, L. H. Sulfonyl fluorides as privileged warheads in chemical biology. Chem. Sci. 2015, 6, 2650–2659; (g) Zhao, Q.; Ouyang, X.; Wan, X.; Gajiwala, K. S.; Kath, J. C.; Jones, L. H.; Burlingame, A. L.; Taunton, J. Broad-spectrum kinase profiling in live cells with lysine- targeted sulfonyl fluoride probes. J. Am. Chem. Soc. 2017, 139, 680–685; (h) Fadeyi, O. O.; Hoth, L. R.; Choi, C.; Feng, X.; Gopalsamy, A.; Hett, E. C.; Kyne, R. E.; Robinson, R. P.; Jones, L. H. Covalent enzyme inhibition through fluorosulfate modification of a noncatalytic serine residue. ACS Chem. Biol. 2017, 12, 2015–2020; (i) Wang, N.; Yang, B.; Fu, C.; Zhu, H.; Zheng, F.; Kobayashi, T.; Liu, J.; Li, S.; Ma, C.; Wang, P. G.; Wang, Q.; Wang, L. Genetically encoding fluorosulfate-L-tyrosine to react with lysine, histidine, and tyrosine via SuFEx in proteins in vivo. J. Am. Chem. Soc. 2018, 140, 4995–4999; (j) Yang, B.; Wu, H.; Schnier, P. D.; Liu, Y.; Liu, J.; Wang, N.; DeGrado, W. F.; Wang, L. Proximity-enhanced SuFEx chemical cross-linker for specific and multitargeting cross-linking mass spectrometry. Proc. Natl. Acad. Sci. U. S. A. 2018, 115, 11162–11167; (k) Martín-Gago, P.; Olsen, C. A. Arylfluorosulfate-based electrophiles for covalent protein labeling: A new addition to the arsenal. Angew. Chem. Int. Ed. 2019, 58, 957–966.
- 18(a) Smith, W. C.; Engelhardt, V. A. Chemistry of sulfur tetrafluoride.1 V. Preparation of sulfur oxytetrafluoride and sulfur hexafluoride by oxidation of sulfur tetrafluoride. J. Am. Chem. Soc. 1960, 82, 3838–3840; (b) Li, S.; Wu, P.; Moses, J. E.; Sharpless, K. B. Multidimensional SuFEx click chemistry: sequential sulfur(VI) fluoride exchange connections of diverse modules launched from an SOF4 hub. Angew. Chem. Int. Ed. 2017, 56, 2903–2908; (c) Gao, B.; Li, S.; Wu, P.; Moses, J. E.; Sharpless, K. B. SuFEx chemistry of thionyl tetrafluoride (SOF4) with organolithium nucleophiles: synthesis of sulfonimidoyl fluorides, sulfoximines, sulfonimidamides, and sulfonimidates. Angew. Chem. Int. Ed. 2018, 57, 1939–1943; (d) Liu, F.; Wang, H.; Li, S.; Bare, G. A.; Chen, X.; Wang, C.; Moses, J. E.; Wu, P.; Sharpless, K. B. Biocompatible SuFEx click chemistry: Thionyl tetrafluoride (SOF4)-derived connective hubs for bioconjugation to DNA and proteins. Angew. Chem. Int. Ed. 2019, 58, 8029 –8033.
- 19(a) Meng, G.; Guo, T.; Ma, T.; Zhang, J.; Shen, Y.; Sharpless, K. B.; Dong, J. Modular click chemistry libraries for functional screens using a diazotizing reagent. Nature 2019, 574, 86–89; (b) Topczewski, J. J.; Liu, E. Double-click enables synthesis of chemical libraries for drug discovery. Nature 2019, 574, 42–43.
- 20 Flower, G. The structure of organized change: a conversation with Kevin Kelly. https://people.well.com/user/bbear/kellyart.html.