Solution-reaction calorimetric study of coordination compounds of rare earth perchlorates with alanine and imidazole
Abstract
Two coordination compounds of rare earth perchlorates with alanine and imidazole, [RE(Ala)n(Im)(H2O)]-(ClO4)3(s) (RE=La, n=3; RE=Nd, n=2), have been prepared and characterized. The standard molar enthalpies of reaction for the following two reactions, LaCl3*7H2O(s) + 3Ala(s)+Im(s)+3NaClO4(s)=[La(Ala)3(Im)(H2O)]-(Cl04)3(S) f 3NaCI(s) + 6H2O(1) (1) and NdCl3 6H2O(s) + 2Ala(s) + Im(s) + 3NaClO4(s) = [Nd(Ala)2(Im)(H2O)]-(ClO4)3(s)+3NaCl(s)+5H2O(1) (2), were determined by solution-reaction calorimetry, at T=298.15 K, as 36.168±0.642 kJ.mol−1 and 48.590±0.934 kJ.mol−1 respectively. From the results and other auxiliary quantities, the standard molar enthalpies of formation of [La(Ala)3(Im)(H2O)](Cl04)3(s) and [Nd(Ala)2(Im)(H2O)] (Cl04)3(s) were derived, ΔfH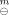 {[La(Ala)3(Im)(H2O)](ClO4)3, s) =(−2984.8 ± 1.0) kJ.mol-l and Δf H
{[La(Ala)3(Im)(H2O)](ClO4)3, s) =(−2984.8 ± 1.0) kJ.mol-l and Δf H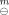 {[Nd(Ala)2(Im)(H2O)]-(ClO4)3,s) =(−2387.8±0.8) kJ.mol−1, respectively.
{[Nd(Ala)2(Im)(H2O)]-(ClO4)3,s) =(−2387.8±0.8) kJ.mol−1, respectively.




