Thermochemistry of the ternary complex Nd(Etndtc)3(phen)
Abstract
The ternary solid complex has been synthesized with sodium diethyldithiocarbamate (NaEt2dtc·3H2O), 1,10-phenanthroline (o-phen·H2O) and hydrated neodymium chloride in absolute ethanol. The title complex was identified as the general formula of Nd(Et2dtc)3(phen) by chemical and elemental analyses. IR spectrum of the complex showed that the Nd3+ coordinated with six sulfur atoms of three NaEt2dtc and two nitrogen atoms of o-phen. It was assumed that the coordination number of Nd3+ is eight. The enthalpy change of liquid-phase reaction of formation, ΔrH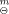 (1), was determined as (-12.274±0.050) kJ·mol−1 at 298.15 K by a microcalorimeter, the enthalpy change of the solid-phase reaction of formation, ΔrH
(1), was determined as (-12.274±0.050) kJ·mol−1 at 298.15 K by a microcalorimeter, the enthalpy change of the solid-phase reaction of formation, ΔrH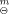 (s), was calculated as (149.069±0.314) kJ·mol−1 on the basis of a thermochemical cycle. The thermodynamics of reaction of formation was studied by changing the temperature of liquid-phase reaction. The constant-volume combustion energy of the complex, ΔcU, was determined as (-18674.22±8.33) kI·mol−1 by a precise rotating-bomb calorimeter at 298.15 K. Its standard enthalpy of combustion, ΔcH
(s), was calculated as (149.069±0.314) kJ·mol−1 on the basis of a thermochemical cycle. The thermodynamics of reaction of formation was studied by changing the temperature of liquid-phase reaction. The constant-volume combustion energy of the complex, ΔcU, was determined as (-18674.22±8.33) kI·mol−1 by a precise rotating-bomb calorimeter at 298.15 K. Its standard enthalpy of combustion, ΔcH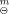 , and standard enthalpy of formation, ΔfH
, and standard enthalpy of formation, ΔfH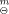 , were calculated as (-18693.43 ± 8.33) kJ·mol−1 and (-47.03 ± 9.17) kJ·mol−1, respectively.
, were calculated as (-18693.43 ± 8.33) kJ·mol−1 and (-47.03 ± 9.17) kJ·mol−1, respectively.




