Stereoselective Synthesis of trans β-Lactams Through Iridium-Catalyzed Reductive Coupling of Imines and Acrylates.
Abstract
For Abstract see ChemInform Abstract in Full Text.
ChemInform Abstract
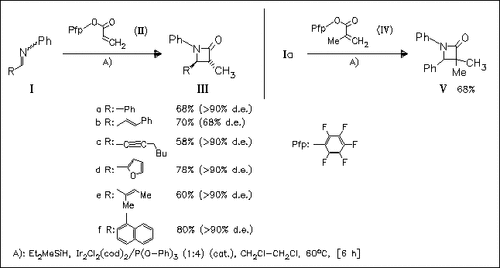
Diethyl(methyl)silane and catalytic amounts of [IrCl(cod)]2/P(OPh)3 are found to be optimal for the title reaction by examination of an array of 96 metal/ligand/silane combinations using a naphthol-based detection assay. In addition, the use of electron-deficient pentafluorophenyl acrylate (II) results in an improved yield compared to the use of phenyl acrylate. In general, > 95% diastereoselectivities for the trans products are obtained, except for cinnamylazetidine (IIIb). The reaction appears to proceed through a reductive Mannich addition—cyclization mechanism. Examination of the substituent effects reveals a linear Hammett correlation for both the N-aryl group on the imine and the aryloxy group on the acrylate