ChemInform Abstract: Conformationally Restricted TRH Analogues: Constraining the Pyroglutamate Region.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
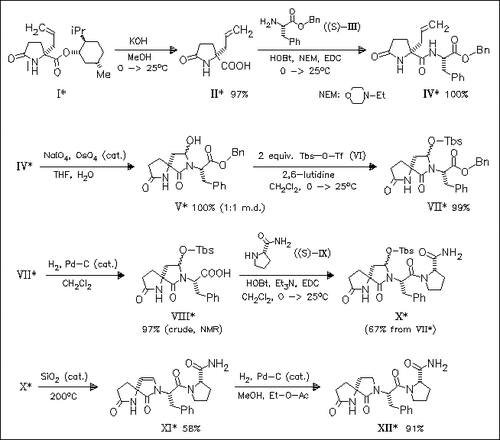
Adding constraints to the pyroglutamate region of TRH leads to a significantly improved method for varying the nature of the constraint. This approach gives rise to the synthesis of analogues bearing an ethylene (XI), an ethane (XII), and a propane bridge in this region. However, altering the original bridge in the pyroglutamate region affords no improvement in the affinity or potency of the analogue for TRH-R.