ChemInform Abstract: The [2 + 2]-Photocycloaddition of Aromatic Aldehydes and Ketones to 3,4-Dihydro-2-pyridones: Regioselectivity, Diastereoselectivity, and Reductive Ring Opening of the Product Oxetanes.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
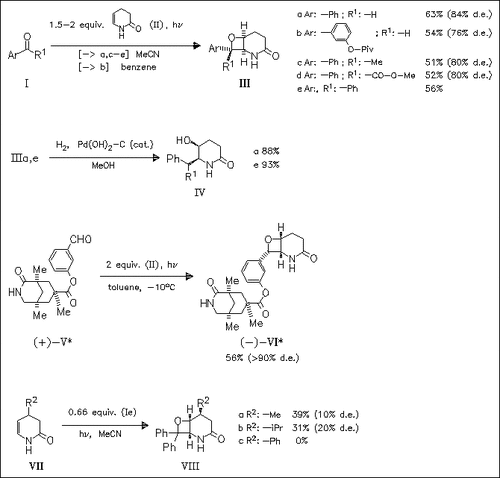
The pyridone (II) smoothly undergoes [2 + 2]photocycloaddition with aromatic aldehydes and ketones to afford fused oxetanes with high regio- and diastereoselectivity. The oxetane ring can easily be cleaved under reduction conditions which opens up a new and efficient route to 2-arylmethyl-3-piperidinols. 4-Substituted dihydropyridones, however, are less suited alkene components in [2 + 2]photocycloaddition reactions. The facial diastereoselectivity in the conversion of the chiral aldehyde (V) is found to be controlled by hydrogen bonds.