ChemInform Abstract: Use of the Vicinal Element Effect for Regiochemical Control of Quinone Substitutions and Its Implication for Convergent Mitomycin Construction.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
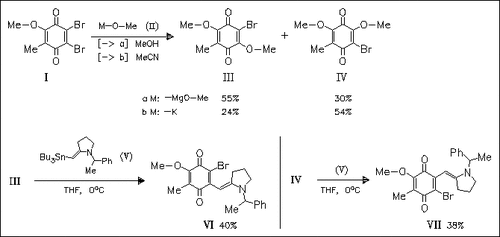
Interestingly, the quinones (III) and (IV) undergo preferred substitution of the methoxy group, which is the poorer leaving group. A vicinal element effect is responsible for this observation. Thus, (IV) is found to be a good candidate for the regioselective construction of mitomycins. Starting from the quinone (I), only the undesired regioisomer is formed.