ChemInform Abstract: Intermolecular Hydroacylation of Acrylate Esters: A New Route to 1,4-Dicarbonyls.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
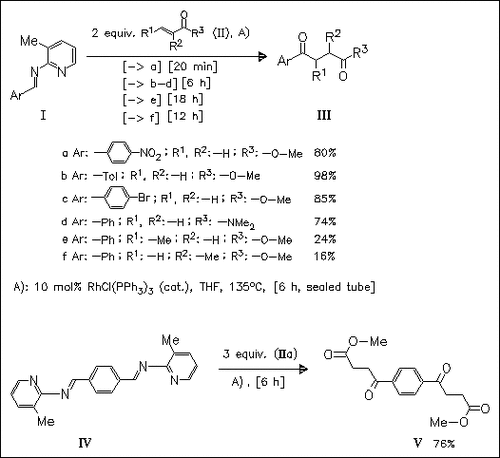
A new route to 1,4-dicarbonyls using Rh(I) mediated hydroacylation reaction between (2-aminopicolyl)imines and acrylate esters is described. The imine component of the reaction can tolerate a range of both electron-withdrawing and electron-donating groups. The enoate component can contain a variety of ester groups, however, the introduction of α- or β-substituents reduces the yields.