ChemInform Abstract: Stereoselective Synthesis of 2-Amino-3-fluoro Bicyclo[3.1.0]hexane-2,6-dicarboxylic Acid.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
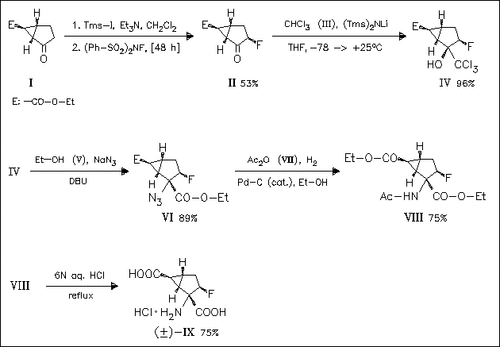
A stereoselective synthesis of the racemic 3-β fluoro analogue (IX) of the conformationally restricted glutamate analogue (+) (LY 354740) is achieved applying a modified Corey—Link reaction to create the amino acid stereogenic center. Under these conditions an azido acyl chloride intermediate is formed which reacts with ethanol to give the inverted azidoester (VI). Hydrogenation of azide (VI) and final hydrolysis afford the bicyclic fluorinate amino acid chlorohydrate salt (IX) with the desired stereochemistry in all five stereogenic centers. The fluorine atom influences the addition of the bulky trichlorocarbonyl anion [(II)→(IV)].