ChemInform Abstract: Stereoselective Synthesis of Enamides by a Peterson Reaction Manifold.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
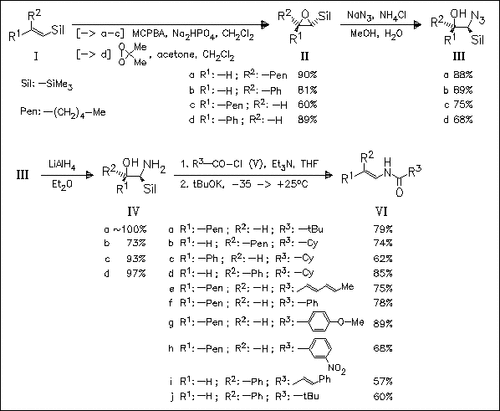
The stereoselective synthesis of enamides is presented, starting with the stereospecific epoxidation of stereochemically defined vinylsilanes (I). Epoxide ring opening and reduction of the introduced azide group furnishes silylated aminoalcohols (IV), which undergo sequential N-acylation and stereospecific Peterson elimination to give enamides (VI). Thus, the double bond geometry of the starting vinylsilanes is restored completely in the enamides. The method is applicable to a wide range of aliphatic and aromatic enamides.