ChemInform Abstract: The Synthesis of β-N-Tosylamino Hydroxylamines via the Ring Opening of N-Tosylaziridines and Their Use in Reverse Cope Cyclizations.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
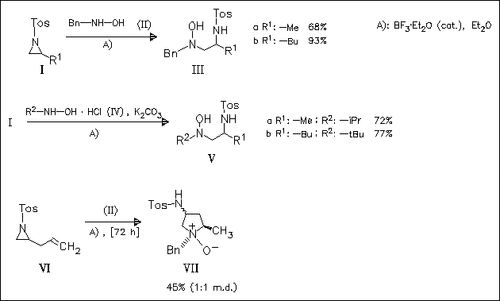
Title compounds (I) are found to undergo regioselective ring opening with hydroxylamines to give β-N-tosylamino hydroxylamines. Suitable substrates, such as (VI), undergo reverse Cope cyclizations to give amino-functionalized pyrrolidine and piperidine N-oxides.