ChemInform Abstract: Cyclization Reactions of 2-Methylbenzenesulfonamides Using N,N-Dimethylcarbamoyl Chloride, N,N-Dimethylthiocarbamoyl Chloride, and N,N-Dimethylsulfamoyl Chloride.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
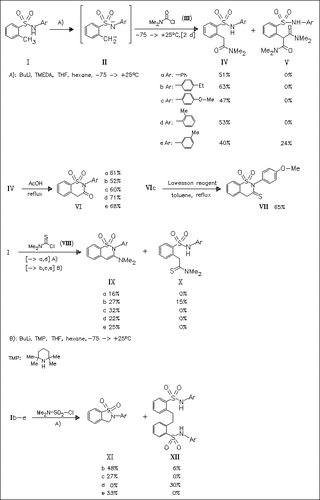
C,N-dianions (II) generated from benzenesulfonamides (I) react with carbamoyl chloride (III) to furnish o-carbamoylmethylbenzenesulfonamides (IV), which cyclize in refluxing AcOH to give the expected benzothiazinone derivatives (VI). Reaction of (II) with thiocarbamoyl chloride (VIII) under similar conditions provides 3-aminobenzothiazine derivatives (IX) instead of the expected benzothiazinethione derivatives of type (VII). On the other hand, reaction of (II) with sulfamoyl chloride affords benzothiazole derivatives (XI) and/or ethylene-bis(benzenesulfonamides) (XII).