ChemInform Abstract: Stereocontrolled Synthesis of Quaternary β,γ-Unsaturated Amino Acids: Chain Extension of D- and L-α-(2-Tributylstannyl)vinyl Amino Acids.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
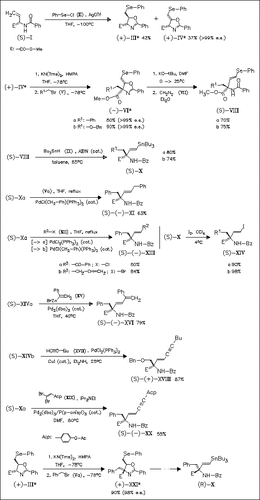
Several D- and L-α-(2-tributylstannyl)vinyl amino acids (AA′s) such as (S)- and (R)-(X) are prepared starting from L-vinylglycine. The reaction sequence involves the installation of a directing β-stereocenter via an episelenonium ion-mediated alkene-masking step and chromatographic separation of the diastereomeric L- and D-vinyl AA synthons (III) and (IV), enolate generation with KN(Tms)2 and alkylative side chain introduction with nearly absolute 1,2-stereoinduction to afford (4S,5R)- and (4R,5S)-4-alkylated oxazolines, oxazoline opening/alkene unmasking under classical E2-type conditions to produce α-[(E)-2-phenylselenovinyl] AA′s, and direct transformation of the latter compounds into the corresponding stannyl derivatives with the tributylstannyl radical. The stannylvinyl AA′s as well as their iodovinyl analogues undergo transition metal-mediated cross couplings of the Stille, Negishi, Sonogashira and Shen types to give AA′s with extended α-(unsaturated alkyl) chains.