ChemInform Abstract: The Preparation of Substituted Isochromans and 1,2,3,4-Tetrahydroisoquinolines by Reduction of Unsaturated Precursors.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
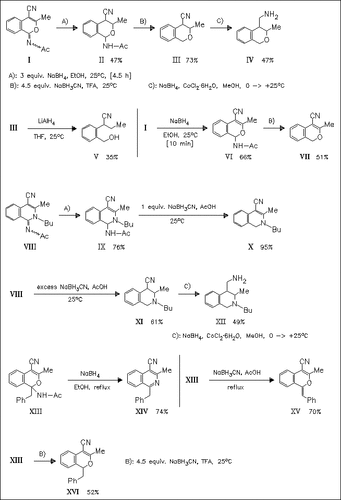
The reductions of isochromene derivatives (I) and (XIII) and of dihydroisoquinoline derivative (VIII) are studied. While the imine function of compounds (I) and (VIII) is readily reduced using NaBH4, the ease of hydrogenation of the 3,4-double bond is different for both derivatives. Generally, under the action of NaBH3CN, reductive loss of the acetylamino group is observed. System NaBH4—CoCl2 is found to reduce the cyano group to provide access to aminomethyl derivatives (IV) and (XII). Reduction of compound (XIII) proceeds less cleanly, and the double bond remains intact irrespective of the reaction conditions used.