ChemInform Abstract: Effects of Halide Ligands and Protic Additives on Enantioselectivity and Reactivity in Rhodium-Catalyzed Asymmetric Ring-Opening Reactions.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
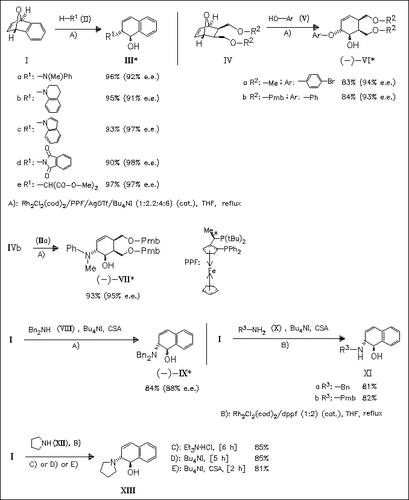
Tricycle (I) and the bicyclic analogue (IV) undergo rhodium/chiral PPF ligand catalyzed ring-opening in the presence of a variety of secondary amines or phenols to give dihydronaphthalenoles and cyclohexenoles with high enantiomeric excesses and yields. The best results are obtained in most cases, if the chloride counterion of the rhodium complex is first removed by addition of AgOTf and the iodide is subsequently added in the form of Bu4NI to generate a rhodium iodide/PPF complex in situ. The ring-opening of (I) with secondary amines such as pyrrolidine under rhodium/achiral ligand catalysis proceeds well either with Et3N×HCl or Bu4NI or Bu4NI/CSA. The other tetrabutylammonium halides give only poor yields. For the reaction with primary amines, good yields are only obtained if Bu4NI/CSA is added.