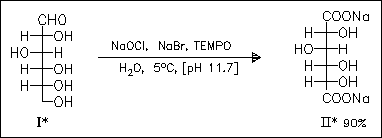ChemInform Abstract: TEMPO-Mediated Oxidation of Maltodextrins and D-Glucose: Effect of pH on the Selectivity and Sequestering Ability of the Resulting Polycarboxylates.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
A ternary oxidation system is applied to selectively oxidize primary alcohol groups of starch hydrolysis products (maltodextrins). The chemoselective oxidation is strongly pH dependent; thus, oxidation of polysaccharides is best achieved at pH 9.5 while oxidation of oligosaccharides require stronger alkaline conditions (pH 11-11.5), followed by the strongest alkaline conditions (pH > 11.5) for D-glucose (I) or D-glucitol. The oxidation of (I) affords glucaric acid (II) with high selectivity and very good yield. Sodium polyglucuronates show interesting sequestering properties with increasing degree of polymerization.