ChemInform Abstract: High-Yielding Staudinger Ligation of a Phosphinothioester and Azide to Form a Peptide.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
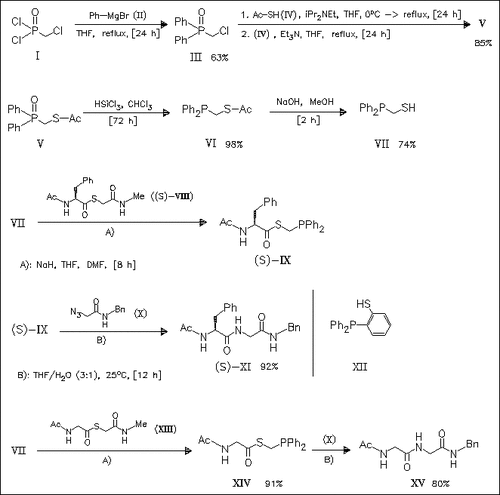
Thioesters such as (IX) and (XIV), prepared from phosphinothiol (VII) and thioesters like (VIII) and (XIII) by transthioesterification (or by DCC coupling with the corresponding acids), undergo Staudinger ligation with azide (X) to give peptides with high yields. In contrast, thioesters derived from thiol (XII) afford the corresponding peptides in significantly lower yields. In addition, the synthesis of the previously unknown thiol (VII) is described.