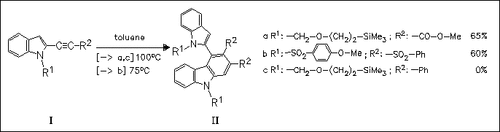
ChemInform Abstract: Cyclodimerization of Indol-2-ylacetylenes. An Example of Intermolecular Enyne—Alkyne Cycloaddition.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
Thermal cyclodimerization of indolylacetylenes (I) proceeds through an enyne—alkyne cycloaddition reaction to give indolylcarbazole derivatives (II). The reaction proceeds only in the presence of electron-withdrawing substituents at the alkyne side chain.