ChemInform Abstract: Scope and Limitations of the [1,2]-Alkylsulfanyl (SMe, SEt and SCH2Ph) and Sulfanyl (SH) Migration in the Stereospecific Synthesis of Substituted Tetrahydrofurans.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
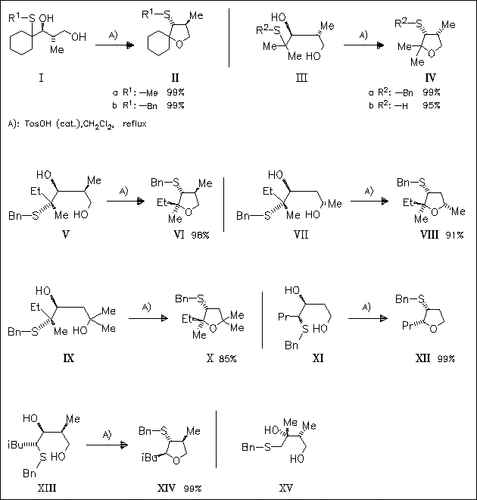
Acid-catalyzed [1,2]-(alkyl)sulfanyl migration in a series of 4-(alkyl)sulfanyl-1,3-diols proceeds regiospecifically with inversion of the configuration at both the migration origin and terminus to give variously substituted tetrahydrofurans as single diastereomers. Alkylsulfanyl and free sulfanyl groups smoothly undergo migration comparable to the phenylsulfanyl group. Secondary and tertiary (alkyl)sulfanyl diols react readily, while primary analogues [cf. (XV)] do not react by [1,2]-sulfanyl shift. The environment of the cyclizing hydroxyl group, in contrast, is without influence.