ChemInform Abstract: Aryl 1-Chloroalkyl Sulfoxides as Acyl Anion Equivalents: A New Synthesis of Vinyl Sulfides, Ketones, and Diketones from Aryl 1-Chloroalkyl Sulfoxides and α,ω-Dichloro-α,ω-disulfinylalkanes.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
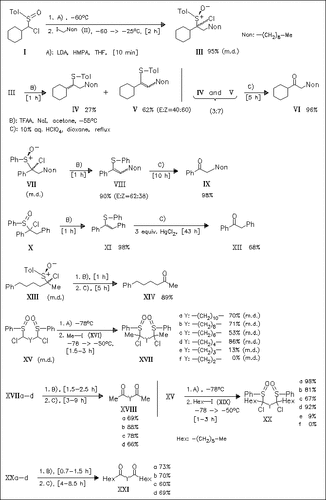
α-Chloroalkyl sulfoxides of type (III), (VII), (X), and (XIII), derived from the alkylation of corresponding sulfoxides with iodoalkanes, give by treatment with TFAA and NaI vinyl sulfides in high yields. These sulfides are converted to ketones by acidic hydrolysis. The procedure is extended to the synthesis of diketones (XVIII) and (XXI). Starting from α,ω-disulfinylalkanes (XV), it is found that alkylation reaction works only well when the length of the carbon chain of disulfinylalkanes is longer than four.