ChemInform Abstract: The Copper-Mediated Cross-Coupling of Phenylboronic Acids and N-Hydroxyphthalimide at Room Temperature: Synthesis of Aryloxyamines.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
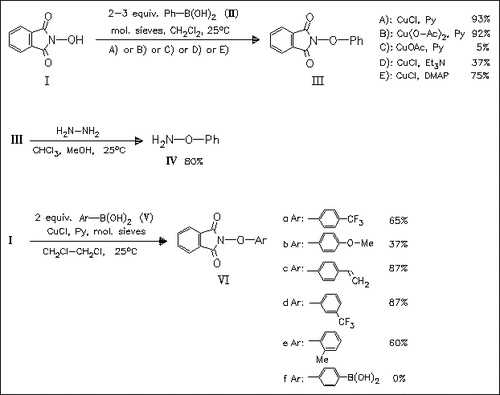
A novel synthetic access to aryloxyamines (III) and (VI) is presented, involving the copper-mediated coupling of arylboronic acids (II) and (V) with N-hydroxyphthalimide (I) in the presence of base. A wide range of electron-donating or electron-withdrawing substituents at the aryl group is tolerated. Removal of the phthalimide group is simply achieved with hydrazine.