ChemInform Abstract: Synthesis and Cytotoxic Activity of a Series of Diacetylenic Compounds Related to Falcarindiol.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
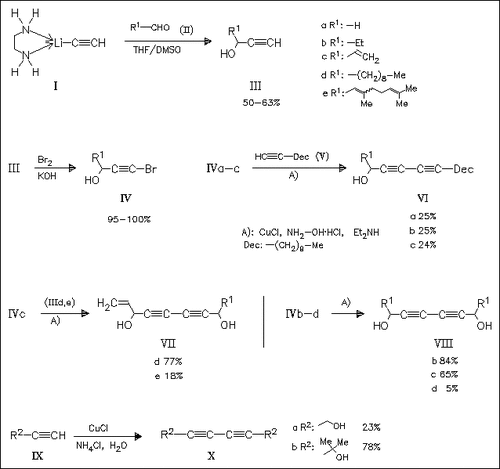
Unsymmetrical diacetylenes (VI) and (VII) are synthesized by modification of the Cadiot—Chodkiewicz coupling reaction of alkynyl bromides (IV) with terminal alkynes. Symmetrical diacetylenes (VIII) and (X) are obtained by using the corresponding alkynyl bromides or by using the Zal′kind modification of the Glaser coupling [(IX)→(X)]. The diacetylenes are tested for in vitro cytotoxic activity against human hepatocellular carcinoma and rat hepatoma cell lines. Compounds (VIc) and (VII) are the most active ones, while symmetrical diacetylenes (VIIIb) and (VIIIc) show intermediate cytotoxicities.