ChemInform Abstract: Substituted Alkyne Synthesis under Nonbasic Conditions: Copper Carboxylate-Mediated, Palladium-Catalyzed Thioalkyne—Boronic Acid Cross-Coupling.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
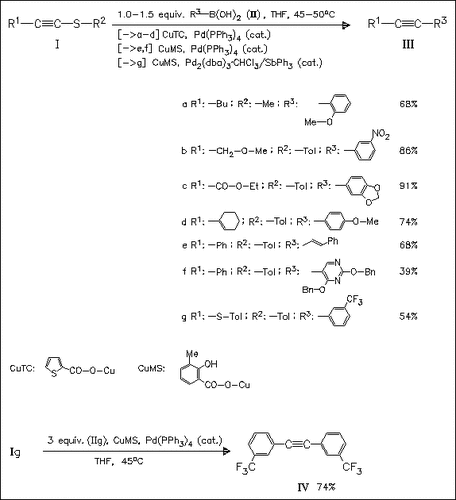
A novel mild and efficient synthesis of internal alkynes (III) is presented, involving the palladium-catalyzed, copper carboxylate-promoted cross-coupling of thioalkynes (I) with boronic acids (II). The method tolerates a wide range of alkenyl, hetaryl, and aryl substituents (containing electron-donating or electron-withdrawing groups) at both the thioalkynes and the boronic acids.