ChemInform Abstract: Intramolecular Hydrosilylation and Silicon-Assisted Cross-Coupling: An Efficient Route to Trisubstituted Homoallylic Alcohols.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
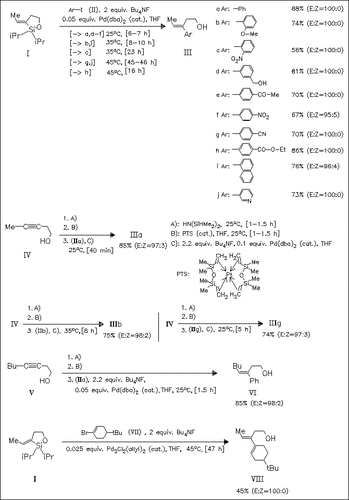
The title compounds are obtained either by palladium-catalyzed cross-coupling of siloxane (I) with aryl iodides (II) [or with bromide (VII)], or by a one-pot arylation of homopropargylic alcohols (IV) or (V), involving silylation of the alcohol group, intramolecular hydrosilylation and cross-coupling with aryl iodides. The first method needs the portionwise addition of aryl iodides to avoid homocoupling, whereas in the one-pot arylation the iodide can be added all at once. Further advantages of the latter method are the superior overall yields and no need to isolate the silyl ethers. The reactions of all halides proceed with high stereospecificity affording the (E)-isomers exclusively or almost exclusively.