ChemInform Abstract: Regiocontrol of Radical Cyclization by Lewis Acids. Efficient Synthesis of Optically Active Functionalized Cyclopentanes and Cyclohexanes.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
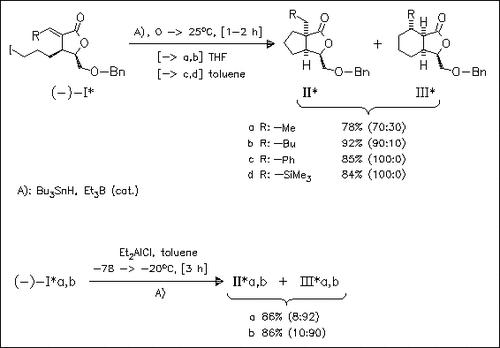
Optically active α-alkylidenelactones (I) undergo a regioselective 5-exo radical cyclization by treatment with Bu3SnH and a catalytic amount of Et3B to give cyclopentane products (II) predominantly or exclusively. If the reaction is performed in the presence of Et2AlCl, only the alkyl-substituted lactones undergo a regioselective 6-endo cyclization to give cyclohexane derivatives (III) predominantly, whereas the reaction of lactones (Ic) and (Id) results in reduction of the olefin and iodo moieties and no cyclohexane derivatives are isolated. In addition, the use of product (IIIa) as starting compound for some carbocycles is demonstrated.