ChemInform Abstract: Studies on the Oxidative Addition Reaction of 1,1-Dibromo-1-alkenes, α-Dehalopalladation, and the Intramolecular Bis(carbopalladation) Reaction of Alkenes. An Efficient Entry to Fused Bicycles.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
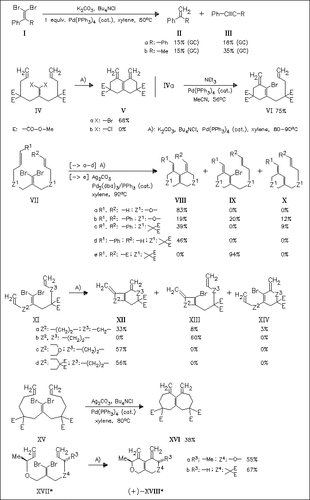
In the presence of stoichiometric amounts of Pd(PPh3)4, the dibromides (I) undergo novel α-dehalopalladation to afford vinylic carbene intermediates which are transformed into alkynes. However, starting from 1,1-dibromo-1-alkenes containing additional C=C double bonds α-dehalopalladation is not observed but cyclic carbopalladation. Depending on reaction conditions and educt structure monocyclization and/or bicyclization products can be obtained. Using this method a number of fused 5,5-, 6,6-, 6,7-, 7,7-bicyclic compounds can be prepared efficiently. In contrast, formation of fused 5,7-, 6,14-, and 6,21-bicyclic compounds is unsuccessful.