ChemInform Abstract: 2-Nitro Analogues of Adenosine and 1-Deazaadenosine: Synthesis and Binding Studies at the Adenosine A1, A2A, and A3 Receptor Subtypes.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
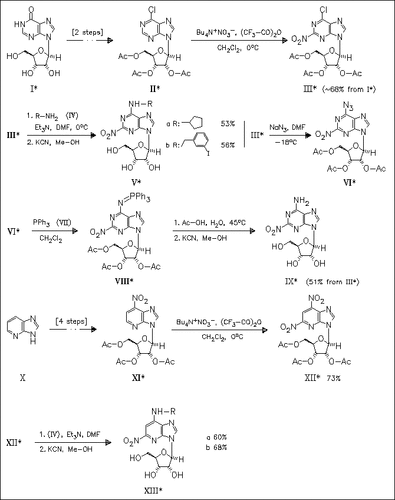
The effect of a nitro group at the 2-position in adenosine analogues (V) and (XIII) together with receptor selective N-6 substituents on the affinity at the adenosine title receptor is investigated. An efficient nitration reagent consists of a mixture of tetrabutylammonium nitrate and trifluoroacetic anhydride. Competing methanolysis of the nitro group of (III) is reduced to a minor process. For the preparation of parent compound (IX), a three-step procedure avoids the use of ammonia. The analogues (V) and (XIII) show good adenosine receptor activity, demonstrating directable selectivity for the A1, A2A, and A3 adenosine receptor subtypes.