ChemInform Abstract: A Highly Efficient and Shortcut Synthesis of Cyclitol Derivatives via Spiro Sugar Orthoesters.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
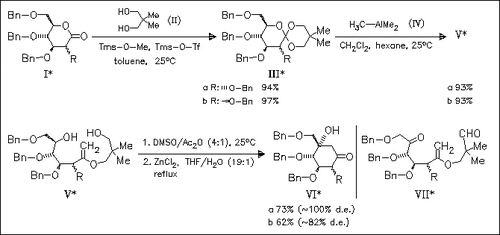
Key steps of the novel synthesis of cyclitol derivatives (VI) are (a) the formation of enol ethers (V) by chemoselective pyran ring opening of sugar ortho esters (III) with methyl anions generated from AlMe3 and (b) the ZnCl2-catalyzed aldol cyclization of keto compounds (VII) in situ generated by DMSO oxidation of compounds (V). The cyclization proceeds with high to excellent diastereoselectivity.