ChemInform Abstract: Conformational Design for 13α-Steroids.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
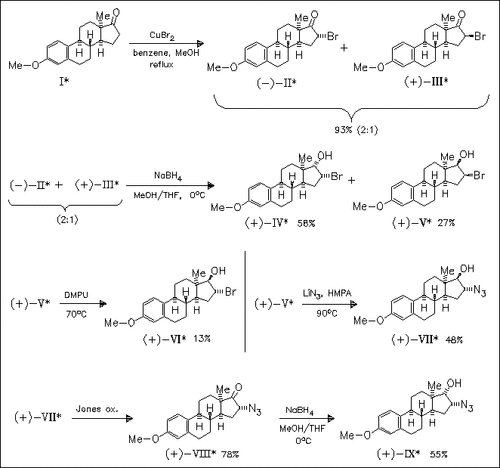
A number of title steroids like (II)—(IX) are prepared as precursors for biologically active compounds and chiral ligands for metal complexation. It is found that the derivatives containing a 17α-substituent or 17-keto group possess the classical steroid conformation, whereas derivatives with a 17β-substituent adopt an atypical C-ring twist-boat and flexible D-ring conformation.