ChemInform Abstract: Asymmetric Total Synthesis of (+)-Equilenin Utilizing Two Types of Cascade Ring Expansion Reactions of Small Systems.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
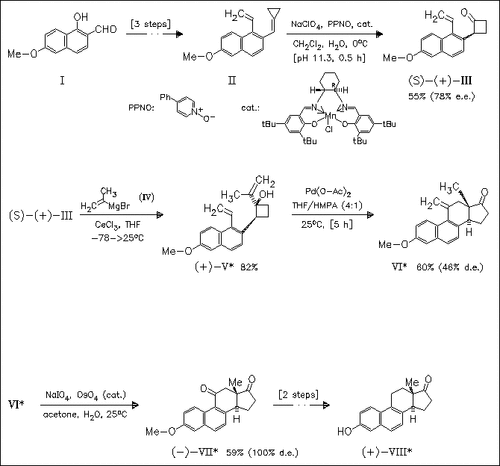
The synthesis of title steroid (VIII) involves an asymmetric epoxidation—ring expansion reaction of cyclopropylidene derivative (II) and palladium-promoted cascade ring expansion—intramolecular insertion reaction of isopropenylcyclobutanol derivative (V) as key steps. In the latter step, the diastereoselectivity significantly depends on the solvents used and in particular trans-product is selectively obtained using THF/HMPA.