ChemInform Abstract: Synthesis of Ionones and Carvone Analogues: Olfactory Properties and Preliminary Toxicity Assays.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
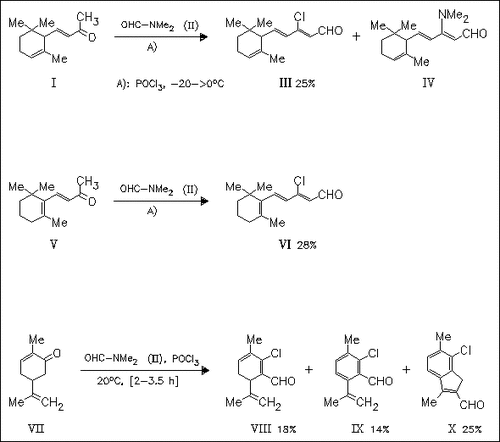
The influence of an aldehyde function in ketones possessing trimethylcyclohexenylalkenone or p-menthone skeletons on the odor is investigated. Thus, the commercially available α- and β-ionones (I) and (V) and carvone (VII) are formylated by the Vilsmeier reagent. Modifying the work-up, compounds (III), (IV), and (VI) are obtained separately with improved yields, while modifying the reaction time of the formylation of carvone (VII) improves the yield of each product. All compounds have peculiar odors which are different from the starting material, with exception of indene (X). Compounds (VIII) and (IX) are the most promising potential perfume ingredients.