ChemInform Abstract: Total Synthesis of a Monocyclofarnesane Dinorsesquiterpenoid Isolated from Mushroom Ingested by Beetle: Selectivity in Solid-State Baeyer—Villiger Reaction.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
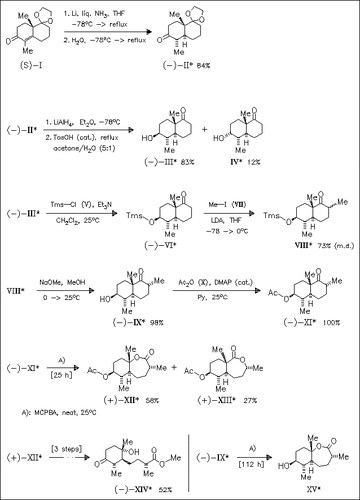
Starting from the known enone (I), the synthesis of intermediate (IX) containing all the requisite stereocenters of the target title compound (XIV) is accomplished in seven steps. The Baeyer—Villiger reaction is best accomplished with acetate protected derivative (XI) instead of free alcohol (IX) to give the desired lactonic compound (XII) and its regioisomer (XIII) in good yields. Final hydrolysis of lactone (XII) followed by Jones oxidation and esterification affords the target compound.