ChemInform Abstract: Chiral Synthons from Carvone. Part 44. An Enantiospecific Approach to Pinguisanes from (R)-Carvone. Total Synthesis of (+)-Pinguisenol.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
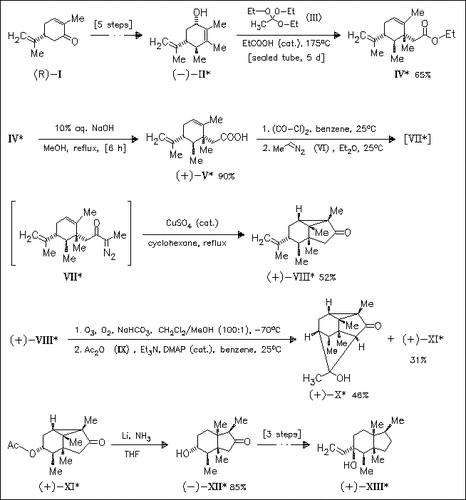
The title compound is prepared by an enantiospecific approach starting from (R)-carvone (I) by employing a Claisen rearrangement, an intramolecular diazo ketone cyclopropanation, a degradation of the isopropenyl group, and a regioselective reductive cyclopropane ring-cleavage sequence. Hydroxy ketone (XII) is finally transformed by Wolff—Kishner reduction, oxidation, and Grignard reaction to furnish the target sesquiterpene.