ChemInform Abstract: Trialkylmanganate Mediated Radical Addition of Triphenylgermane to Carbon—Carbon Multiple Bonds.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
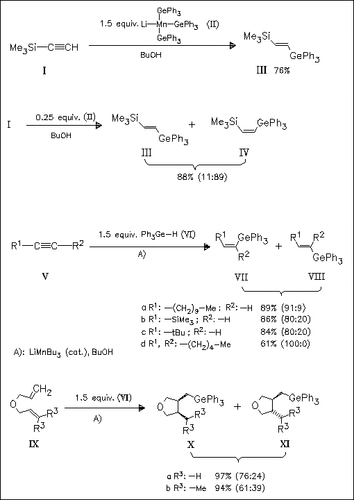
The addition of triphenylgermane to carbon—carbon multiple bonds via the corresponding manganese ate complex, tris(triphenylgermyl)manganate (II) or via a catalytic pathway using tributylmanganate is investigated. The stereochemical outcome of the former reaction depends on the molar ratio between the manganese reagent and the acetylenic compound. An excess of the reagent produces exclusively the corresponding E-alkene (III) whereas the Z-isomer (IV) becomes the main product with excess acetylenic compound. It is assumed that the reaction proceeds in a radical pathway and tributylmanganate(II) is an initiator. Thus, the treatment of C—C multiple bonds with triphenylgermane in the presence of catalytic amounts of tributylmanganate provides the corresponding addition products in high yields.