ChemInform Abstract: Domino Michael—Seleno Pummerer Type Reaction (Additive Seleno Pummerer Reaction).
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
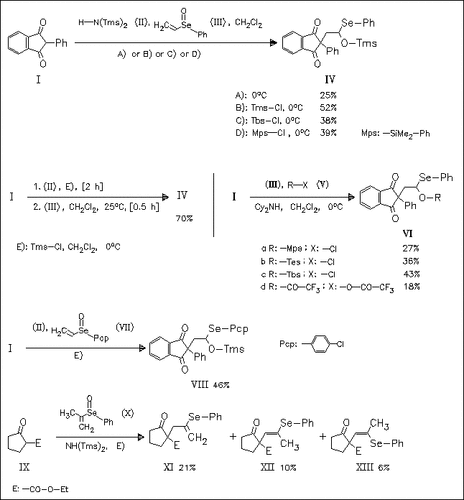
1,3-Dicarbonyl compounds such as (I) and (IX) undergo an additive seleno Pummerer type reaction with various vinyl(aryl) selenoxides in the presence of an amine and a chlorosilane to give the corresponding siloxymethyl(aryl)selenides such as (IV), (VI), and (VIII) (the yields for further seven analogous products are given without experimental conditions). It is found that either the Tms group of HN(Tms)2 is introduced in the presence of silanes or the silyl groups of chlorosilanes are introduced in the absence of HN(Tms)2 and the presence of Cy2NH. Selenides (XI), (XII), and (XIII) derived from the reaction with selenoxide (X) are formed by TmsOH elimination.