ChemInform Abstract: Facile Syntheses of Building Blocks for the Construction of Phosphotyrosine Mimetics.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
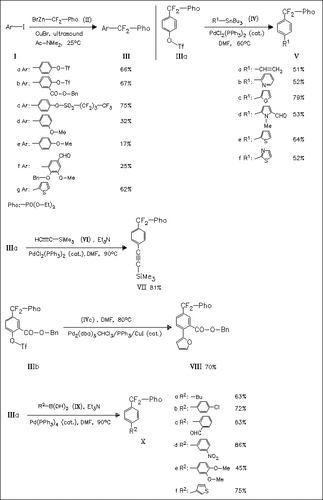
The coupling described by Yokomatsu and Shibuya for 1,4-diiodobenzene with phosphoryldifluoromethyl organozinc reagent (II) is successfully applied to various iodides (I) (CuBr, sonication) to afford coupling products (III). The best yields are obtained for sulfonates (IIIa)—(IIIc). Triflates (IIIa) and (IIIb) are found to undergo Stille and Suzuki coupling reactions to give a variety of phosphoryldifluoromethyl arenes and biaryls in moderate to high yields. If the triflate (IIIa) is replaced by the corresponding iodide in the Stille coupling reactions, the yields of products (V) in most cases are equal or lower [except for (Va), isolated in 84% yield].