ChemInform Abstract: Type-Two Intramolecular Diels—Alder Reactions of Pyrazolo-o-quinodimethanes.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
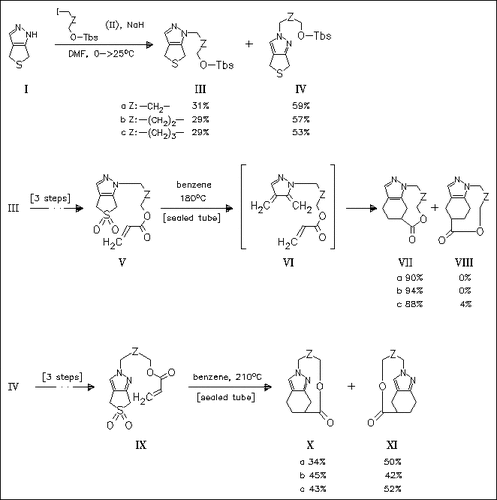
Thermolysis of the pyrazolosulfolenes (V) and (IX) leads to the corresponding o-quinodimethanes which spontaneously undergo type-two intramolecular Diels—Alder reaction to afford two- and three-atom bridged tricyclic pyrazoles which are otherwise difficult to prepare. Depending on the N-substitution position the temperature required for extrusion of SO2 and the regioselectivity of cyclization are influenced substantially.