ChemInform Abstract: Formation and Destruction of Diazine Ring under Conditions of the Vilsmeier—Haack Formylation of 4-Dialkylaminonaphthalic Acid Derivatives.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
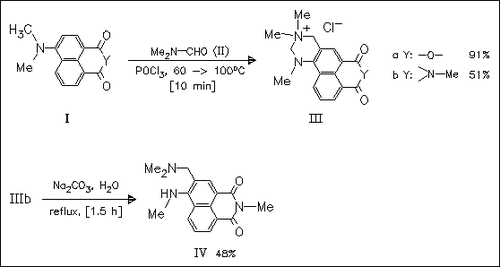
The 4-dimethylaminonaphthalic acid derivatives (I) react with DMF under Vilsmeier—Haack conditions to yield the quaternary salts (III) instead of expected carbaldehydes. The quaternized diazine ring is hydrolyzed in alkaline aqueous media to diamines [cf. (IV)].