ChemInform Abstract: Synthesis, Structure, and Properties of Hexaaryl[3]radialenes.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
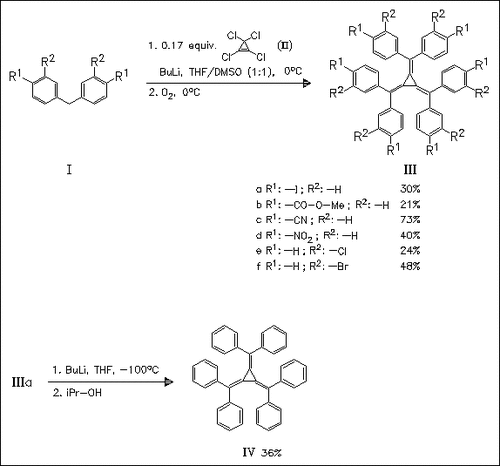
A number of hexaaryl[3]radialenes (III) are synthesized in moderate to good yields by reaction of tetrachloropropene with the carbanions of substituted diphenylmethanes followed by oxidation with oxygen. Especially, diarylmethanes with high acidity afford good yields of radialenes. Except for hexanitro compound (IIId) all radialenes are fairly stable substances.