ChemInform Abstract: Cyclopropane-Shift Type Reaction of Diaryl(2-halogenocyclopropyl)methanols Promoted by Lewis Acids.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
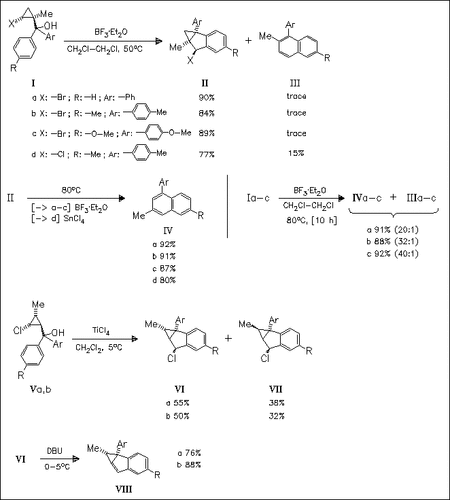
A new, novel Lewis acid promoted cyclopropane-shift reaction of the cyclopropanes (I) and (V) is observed. Thus the reaction of (I) and (V) results in the formation of the tricyclic compounds (II), (VI) and (VII), which are transformed into the naphthalenes (IV) and arylindenes like (VIII), respectively. Alternatively, naphthalene derivatives (IV) are also prepared in a direct, one-pot cyclopropane-shift type benzannulation of (I). These reactions represent an interesting example of C-bond fission across a cation.