ChemInform Abstract: Halogen—Magnesium Exchange via Trialkylmagnesates for the Preparation of Aryl- and Alkenylmagnesium Reagents.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
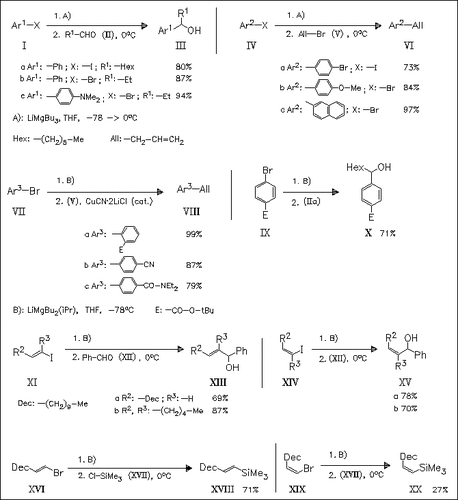
Various aryl or alkenyl iodides or bromides give, under treatment with trialkylmagnesates R3MgLi, derived from RMgX and two equivalents alkyllithium, the corresponding intermediate organomagnesium reagents, which can be trapped with various electrophiles (alkyl halogenides, aldehydes, ketones, silyl chloride). It is found that iPrBu2MgLi is more reactive than Bu3MgLi in the reaction of aryl bromides bearing an ester, amide or nitrile group with aldehydes. In addition, a catalytic amount of CuCN is necessary to facilitate the allylation of these bromides at -78°C. The application of the new title method toward alkenyl halides proceeds with complete retention of configuration of the double bond.